ADEPT®

The Impact of Adhesions
#1 Cause of Secondary Infertility in Women
Adhesions are the number one cause of secondary infertility in women.1
34.5% of Women Readmitted into Hospital for Further Surgery
Following initial gynecologic surgery, up to one in three patients were readmitted for a problem potentially related to adhesions or for further surgery potentially complicated by adhesions over a 10-year period.2
Lysis of Adhesions at Reoperative Surgery
Is associated with inadvertent organ injury, prolonged operative time, an increased risk of post-operative complications, and higher costs3

The Only FDA-Approved Fluid-Based Solution for Gynecological Adhesion Reduction 4
ADEPT® is a non-viscous, colorless liquid used for intraperitoneal administration to reduce adhesions in gynecological laparoscopic adhesiolysis. The solution contains 4% icodextrin and does not impair viewing or the handling of tissue.4
A Simple and Effective Solution
Direct and Fast-Acting Application
As a liquid, ADEPT® is administered directly and rapidly to the site through a laparoscopic port during surgery5
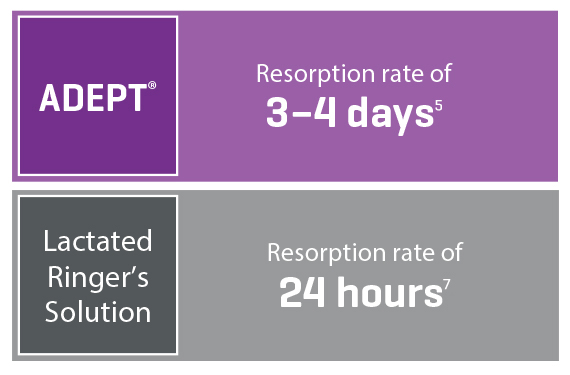
Broad Coverage and Optimized Resorption
Ingredient icodextrin slows the resorption time, whereas regular fluids would be resorbed much faster6
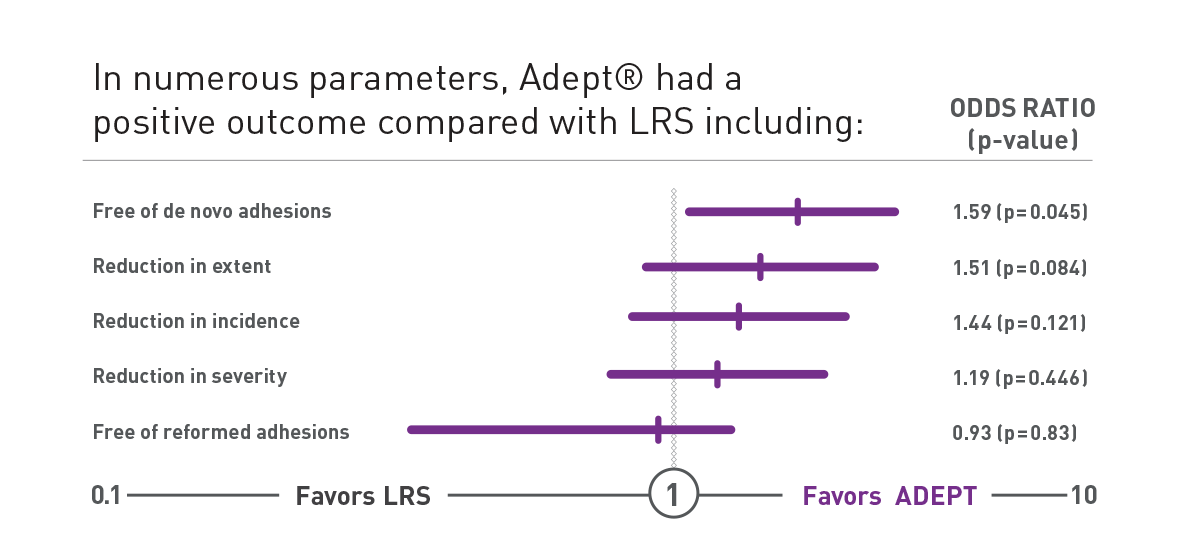
Proven Clinical Success with the PAMELA Study
Double-blind randomized controlled trial comparing ADEPT® (n=203) and Lactated Ringer's Solution (n=199) during laparoscopic gynecological surgery8
ADEPT® Adhesion Reduction Solution
[4% Icodextrin] Indications
ADEPT® Adhesion Reduction Solution is indicated for use intraperitoneally as an adjunct to good surgical technique for the reduction of post-surgical adhesions in patients undergoing gynecological laparoscopic adhesiolysis.
Important Risk Information for ADEPT®
ADEPT® Solution is for direct intraperitoneal administration only. NOT for intravenous (IV) administration.
ADEPT® is contraindicated in patients with known or suspected allergy to cornstarch based polymers e.g. icodextrin, or with maltose or isomaltose intolerance, or with glycogen storage disease.
ADEPT® is contraindicated in laparotomy, in cases involving bowel resection or repair, or appendectomy and in surgical cases with frank abdomino-pelvic infection.
There have been rare reports of sterile peritonitis following the use of icodextrin.
Leakage of ADEPT® from port sites may lead to wound healing complications; meticulous fascial closure may reduce leakage through laparoscopic port sites post-operatively.
There have been rare reports of hypersensitivity reactions, pulmonary edema, pulmonary effusion and arrhythmia.
Anaphylaxis has been reported in a few patients.
Maltose metabolites of icodextrin may interfere with blood glucose measurement in diabetic patients who use rapid blood glucose systems that are not glucose specific.
In the pivotal study, the most frequently occurring treatment related adverse events between surgeries were post procedural leaking from port sites, labial, vulvar or vaginal swelling and abdominal distention.
Rx Only: For safe and proper use of this device, please refer to full Instructions For Use.