Elevating Patient Care: Addressing Spine Surgery Challenges
Patient profiles are changing, increasing the risk of bleeding and healing complications in spine surgery.1
Between 2015 and 2050,
the proportion of the world's population over 60 years will nearly double from 12% to 22%2
30%
of the US population use low-dose aspirin for cardiovascular disease prevention3
~2/3
of US surgical patients are treated with anticoagulants and/or antiplatelets4
1 in 3 women and 1 in 5 men
over age 50 will experience osteoporosis fractures in their remaining lifetimes5
More than 2 in 5 adults
have obesity6
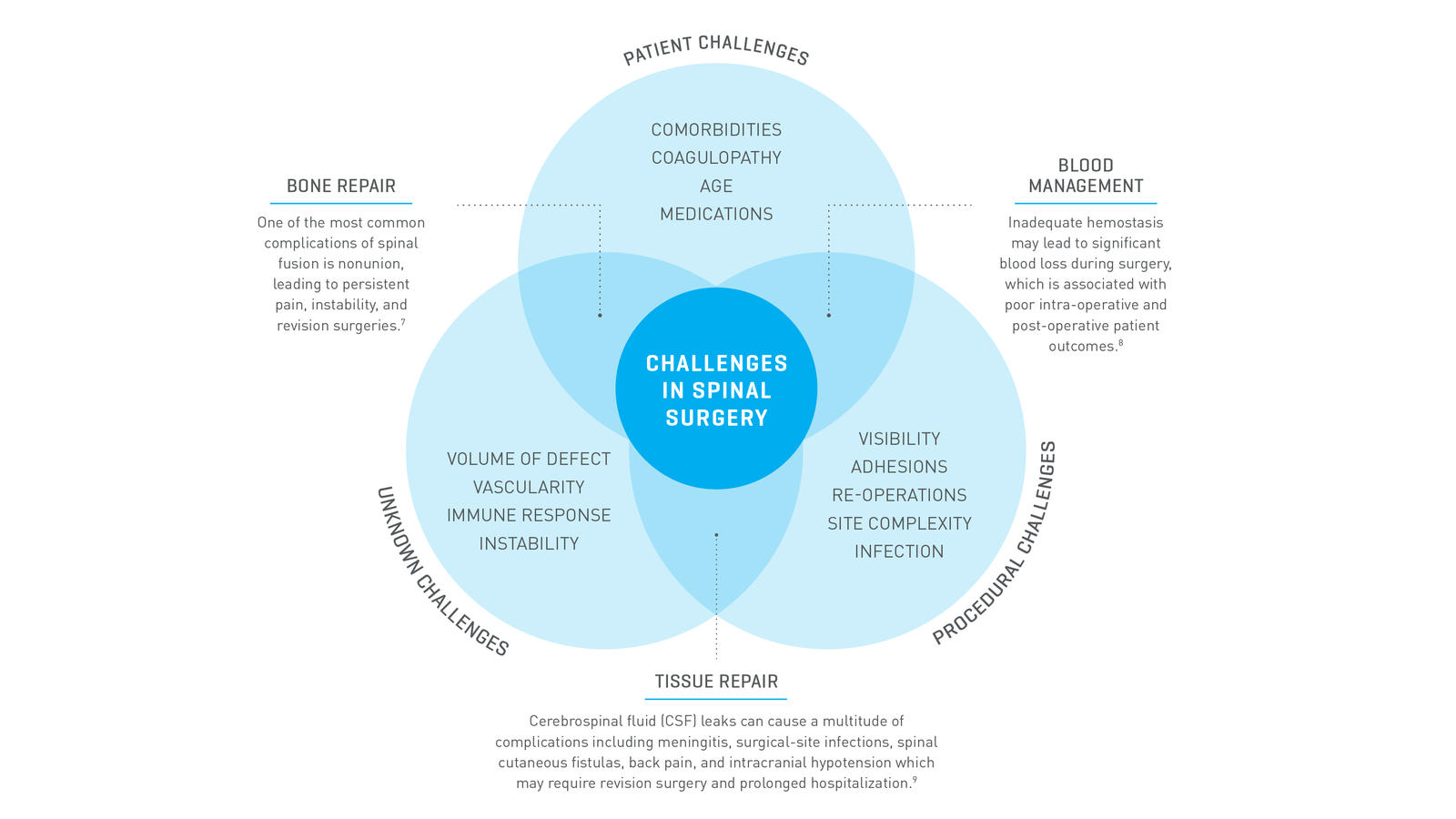
Each spine procedure provides unique challenges that can cause complications for the healing process.7-9
There is a high incidence of bleeding and healing-related complications in spine surgery.
Approximately 30%
of spine surgery patients require blood transfusions.10
Approximately 141%
higher cost associated with procedures involving CSF leaks in neurosurgery.11
Up to 13.9%
operative nonunion rate with increased risk of nonunion for constructs that include L5-S1 and ≥3-level fusions.12
On average, there is an up to 83%
increase in total cost of care for spine surgery patients with bleeding related complications, according to a U.S. case report analysis.13
Revision rates of up to 33.8%
in spine surgeries present a major challenge for surgeons and the healthcare system.14
How to Optimize Conditions for Successful Fusion: A Patient-Centric Strategy

Achieving Fusion
As a patient’s osteogenic capacity decreases due to aging and comorbidities, reliance on the graft material increases.16-17 Fusion rates can potentially be improved by choosing the most effective bone graft that actively promotes or enhances the regeneration process.17

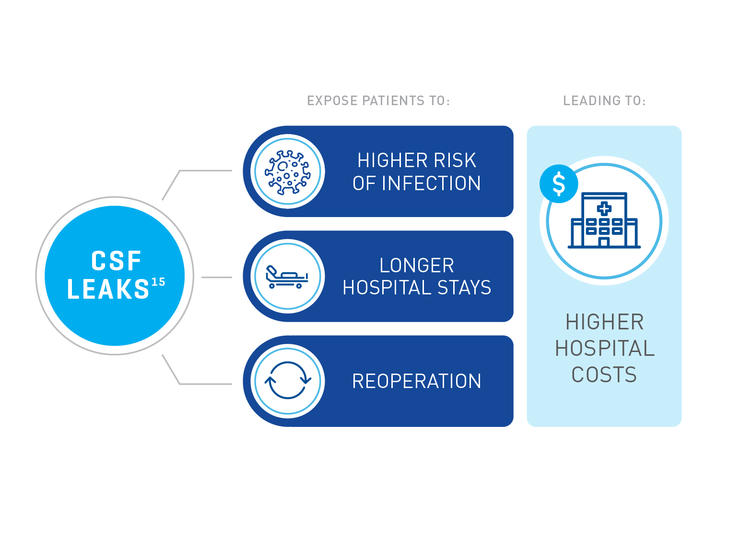
Preventing and Fixing CSF Leaks
Significant bleeding can result in loss of visualization leading to nerve injury or dural tears.18 Visualizing the anatomy during spine surgery is critical to an efficient and successful surgery.18
Watertight dural closure may reduce or avoid the complication of CSF leak19, decreasing the likelihood of surgical site infections and revisions surgeries, reducing hospital stays.15

Achieving Hemostasis
Understanding your patient’s coagulation factors preoperatively and intraoperatively assessing the grade of bleed you encounter can influence your choice in hemostat.1
A hemostatic strategy can be instrumental in minimizing blood loss and transfusions in spinal surgery and neurosurgery.20-21