Navigating Challenges in Cardiac Surgery
Patient profiles are changing; increasing the risk of bleeding complications in cardiac surgery.1
30%
of the US population use low-dose aspirin for Cardiovascular disease prevention.2
~2/3
of surgical patients are treated with anticoagulants and/or antiplatelets.*3,4
*Based on Premier Database data
1 in 3
adults will be diagnosed with cancer in their lifetime.5
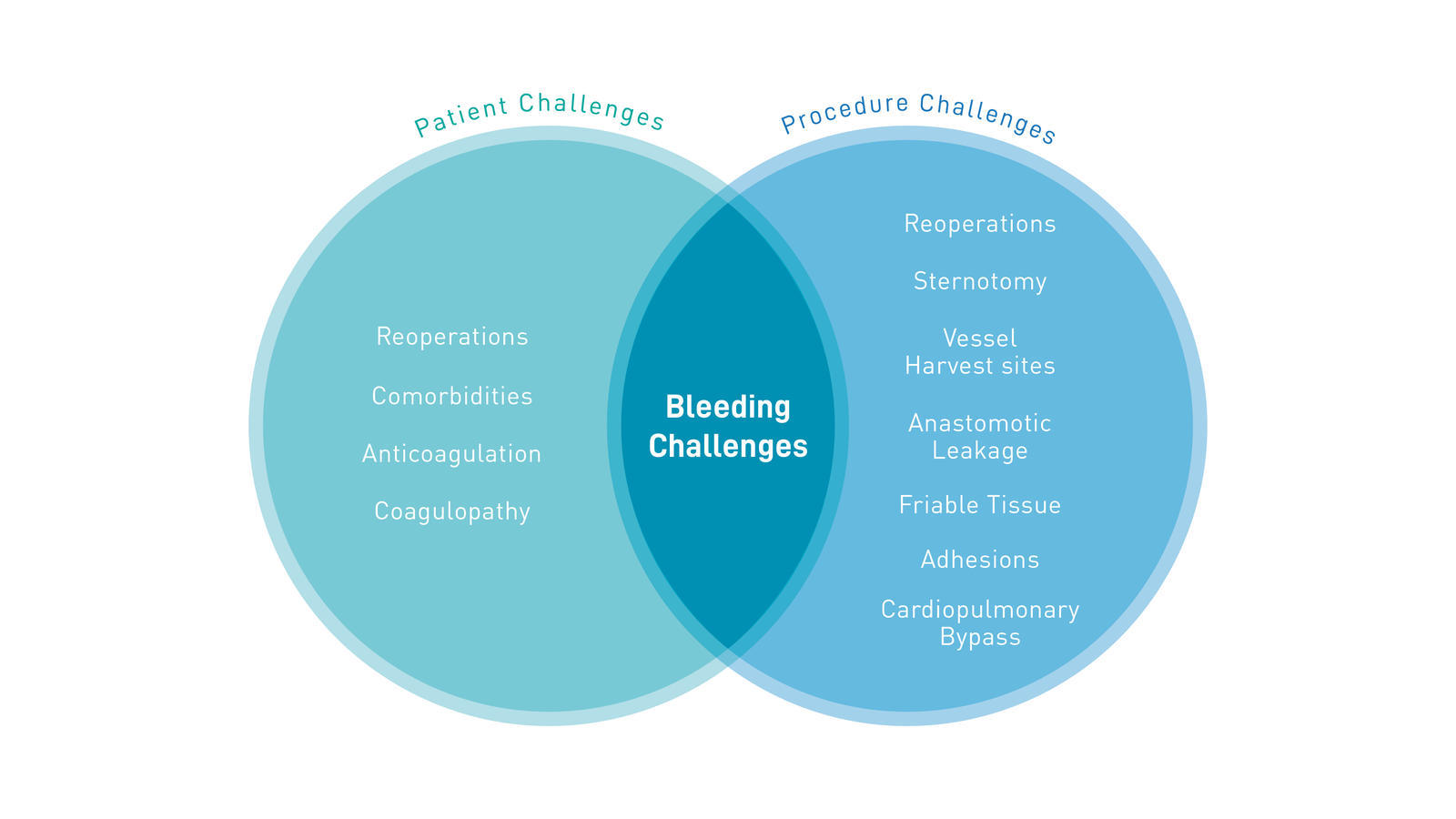
Changing patient profiles result in additional comorbidities, which increase bleeding-related complications in cardiac surgery
There is a high incidence of bleeding-related complications in cardiac surgery
The risk of bleeding in cardiac surgery is especially high due to the invasiveness of procedures, need for anticoagulation, and exposure to cardiopulmonary bypass.6
Approx 20%
of annual U.S. blood transfusions are accounted for in cardiovascular procedures.7
~1/3
of all cardiovascular surgery patients will receive blood transfusions.8,9
Up to 14%
of U.S. cardiovascular surgery patients will require reoperation for bleeding.10
Risks of excessive bleeding

Failure to achieve and maintain hemostasis may lead to excessive bleeding, thereby complicating surgery and increasing the risk of morbidity and mortality.
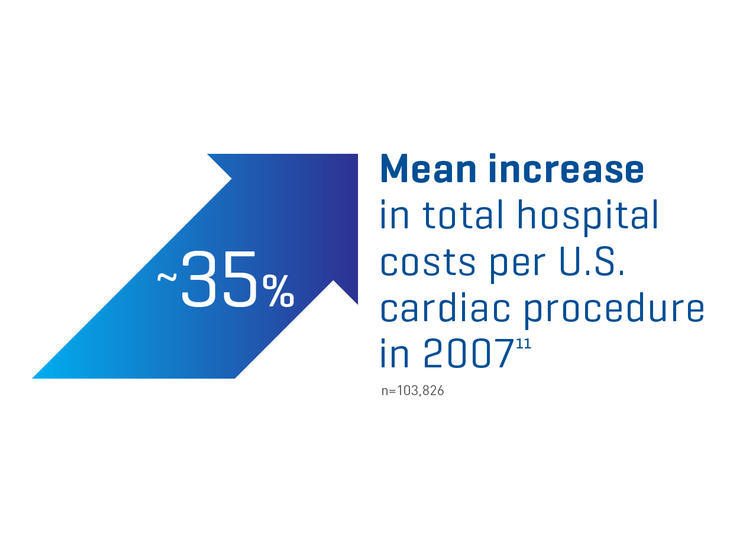
Longer surgery time12
Increased usage of blood products12
Contribute to postoperative complications12
Extended length of hospital stay12
Keeping patients at the core of cardiac surgery strategy

Preventing excessive blood loss
Understanding your patient’s coagulation factors preoperatively and intraoperatively assessing the grade of bleed you encounter can influence your choice in hemostat.12 A hemostatic strategy can be instrumental in minimizing blood loss in cardiovascular surgery.8,9

Achieving sealing
Achieving a strong and durable seal may reduce blood transfusions and drainage in cardiovascular surgery.14,15