VASCU-GUARD
NOW IN A POUCH PRESENTATION
With 25+ years of clinical history, VASCU-GUARD Vascular Repair Patch has demonstrated high levels of biocompatibility, resistance to suture line bleeding, and low adverse event rates supporting overall patient healing outside of the OR.1,2,4,8
See how the new VASCU-GUARD pouch presentation will bring operational and workflow efficiencies to your practice, without compromising safety and effectiveness.3

From Jar to Pouch
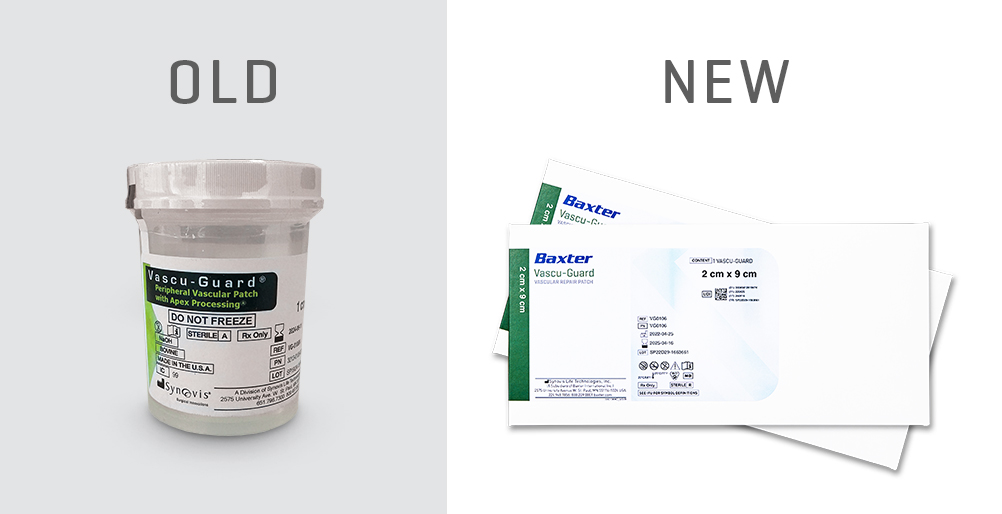
With the implementation of the new packaging, the patch will now be terminally sterilized via gamma irradiation and come in a double-pouch system. The inner pouch maintains the bovine pericardium patch’s moisture while packaged, and it doesn’t contain any water or solution.3
Why the Change?3
Operational Efficiency
Operational and workflow benefits without impacting safety and effectiveness of the vascular repair patch
No Spills
No potential for solution spills when opening the package
Environmentally Friendly
No storage solution to dispose of
Packaging Familiarity
Commonly found in OR products
Shipping Benefit Potential
Reduced potential of product freezing in transit
How to Order
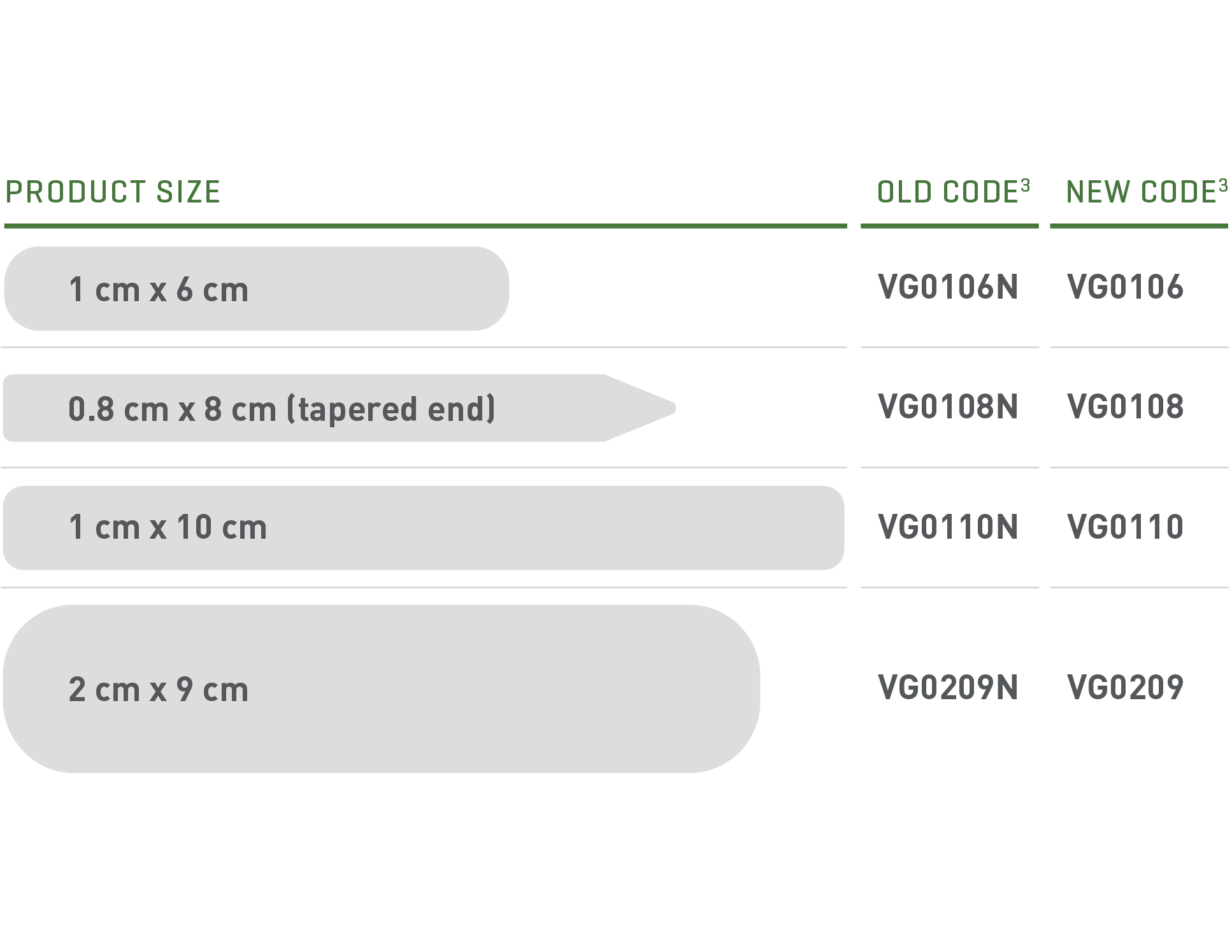
Update Your Ordering Codes3
Ordering numbers have changed for all 4 sizes of VASCU-GUARD. Please ensure the new codes are updated in your Electronic Ordering Systems.
Schedule an In-service Training
This video will guide you through the opening, inspection and rinse procedure steps for the pouch-packaged VASCU-GUARD to help you plan for your live in-service training.
Contact your Baxter Sales Representative to schedule training or complete the form here.
Frequently Asked Questions
Important Risk Information
VASCU-GUARD INDICATIONS FOR USE
VASCU-GUARD Vascular Repair Patch is used in peripheral vascular reconstruction including the carotid, renal, iliac, femoral, profunda and tibial vessels and arteriovenous access revisions.
CONTRAINDICATIONS
VASCU-GUARD is not designed, sold or intended for use except as indicated.
Do not use VASCU-GUARD in patients with known sensitivity to bovine material.
WARNINGS
Failure to rinse the product may result in a sterile inflammatory reaction in the adjoining host tissue.
The product must remain moist at all times.
Do not place outer pouch in sterile field as outside of pouch is not sterile.
Do not use if any folds, creases, or wrinkles are observed in VASCU-GUARD patch.
Do not use if product has been exposed (1) to solutions above room temperature or (2) to chemicals, antibiotics, or substances other than specifically addressed in these instructions as the characteristics of VASCU-GUARD may change or be compromised.
Antimycotics (anti-fungals) must not come in contact with VASCU-GUARD as they are believed to alter the cross-linked characteristics of tissue fixed in aldehyde preparations.
Do not resterilize.
Product is single use only.
Failure to observe warnings may result in surgical infection.
Do not substitute one Synovis product for another.
Clinical experience with glutaraldehyde fixed porcine xenograft heart valves indicates that fixed tissue may be subject to late attack by the body and subsequent tissue deterioration. In like manner, the glutaraldehyde fixed bovine pericardium may be subject to late deterioration. The benefits of the use of this tissue in cardiovascular repair or repair of soft tissue deficiencies must be weighed against the possible risk of aneurysm, hemorrhage, or patch weakening resulting from tissue deterioration.
The patch is not indicated for the construction or replacement of total valves or conduits.
Rinse surgical gloves to remove glove powder prior to touching VASCU-GUARD.
Rx Only. For safe and proper use of this device refer to the complete Instructions for Use.
