Artiss [Fibrin Sealant (Human)]: The Use of Fibrin Sealants for Skin Grafts in Burn Surgery
O.R. Insights Blog
Artiss [Fibrin Sealant (Human)]: The Use of Fibrin Sealants for Skin Grafts in Burn Surgery
The treatment of burn injuries with skin grafts can be traced back to 750-800 BC with the earliest documented use by the surgeon Sushruta.1 Autologous skin translocation, also known as “skin grafting,” has played an important role in burn wound management and has a rich history of its own. 1 In fact, some of the oldest known medical texts describe these ancient methods of skin translocation in detail. 1 Tangential excision and autologous skin graft coverage is a foundational principle in burn surgery.2 In terms of covering the excised wounds, autologous split-thickness skin grafts have evolved as being optimal for full-thickness dermal defect coverage.2 Over the last decades, the use of fibrin sealant has gained popularity as a graft fixation method.2
This article will provide an overview of Artiss [Fibrin Sealant (Human)], a slow-polymerizing fibrin sealant that consists of two plasma-derived components: a sealer protein solution and a thrombin solution, by exploring its mechanism of action, key clinical findings, and providing guidance on use.3 Whether you're already familiar with this fibrin sealant as a skin graft adhesion method, or want to learn more, Dr. Steven Kahn† of the Medical University of South Carolina, will delve into the role it has played in burn surgery, exploring its impact on enhancing graft fixation and patient outcomes.
Artiss [Fibrin Sealant (Human)] is a fibrin sealant indicated to adhere autologous skin grafts to surgically prepared wound beds resulting from burns in adult and pediatric populations greater than or equal to 1 year of age. Artiss [Fibrin Sealant (Human)] is indicated to adhere tissue flaps during facial rhytidectomy surgery (face-lift).
Artiss [Fibrin Sealant (Human)] is not indicated as an adjunct to hemostasis.
Click here for the Indications and Important Risk Information and accompanying Prescribing Information.
Introduction
Over the years, mortality and morbidity in burn surgery have improved significantly.2 Nevertheless, besides burn resuscitation and fluid replacement, the concept of tangential excision and immediate coverage remains rule number one in the approach of a burn patient.2 In terms of covering the excised wounds, autologous split-thickness skin grafts have evolved as being optimal for full-thickness dermal defect coverage.2
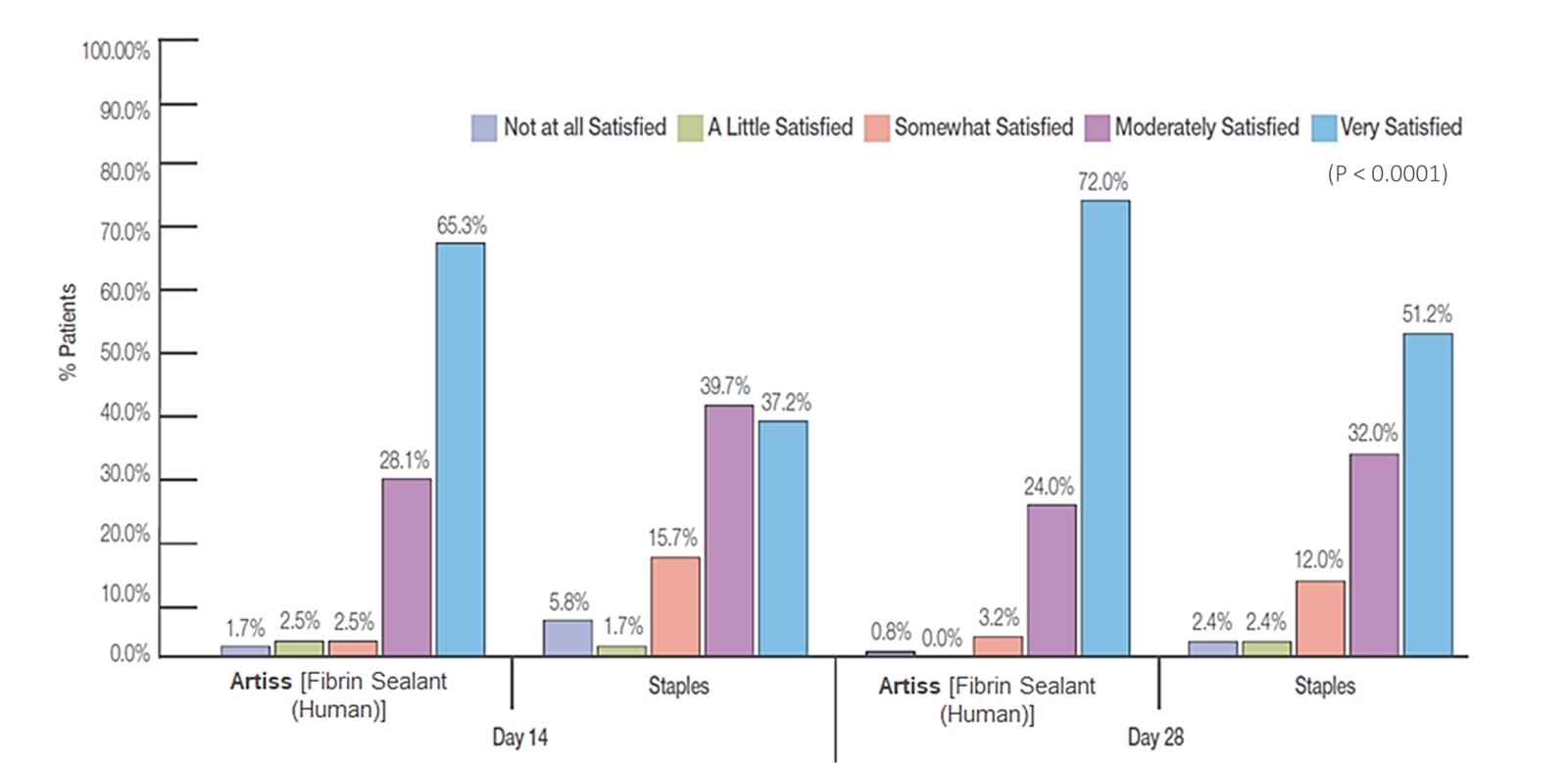
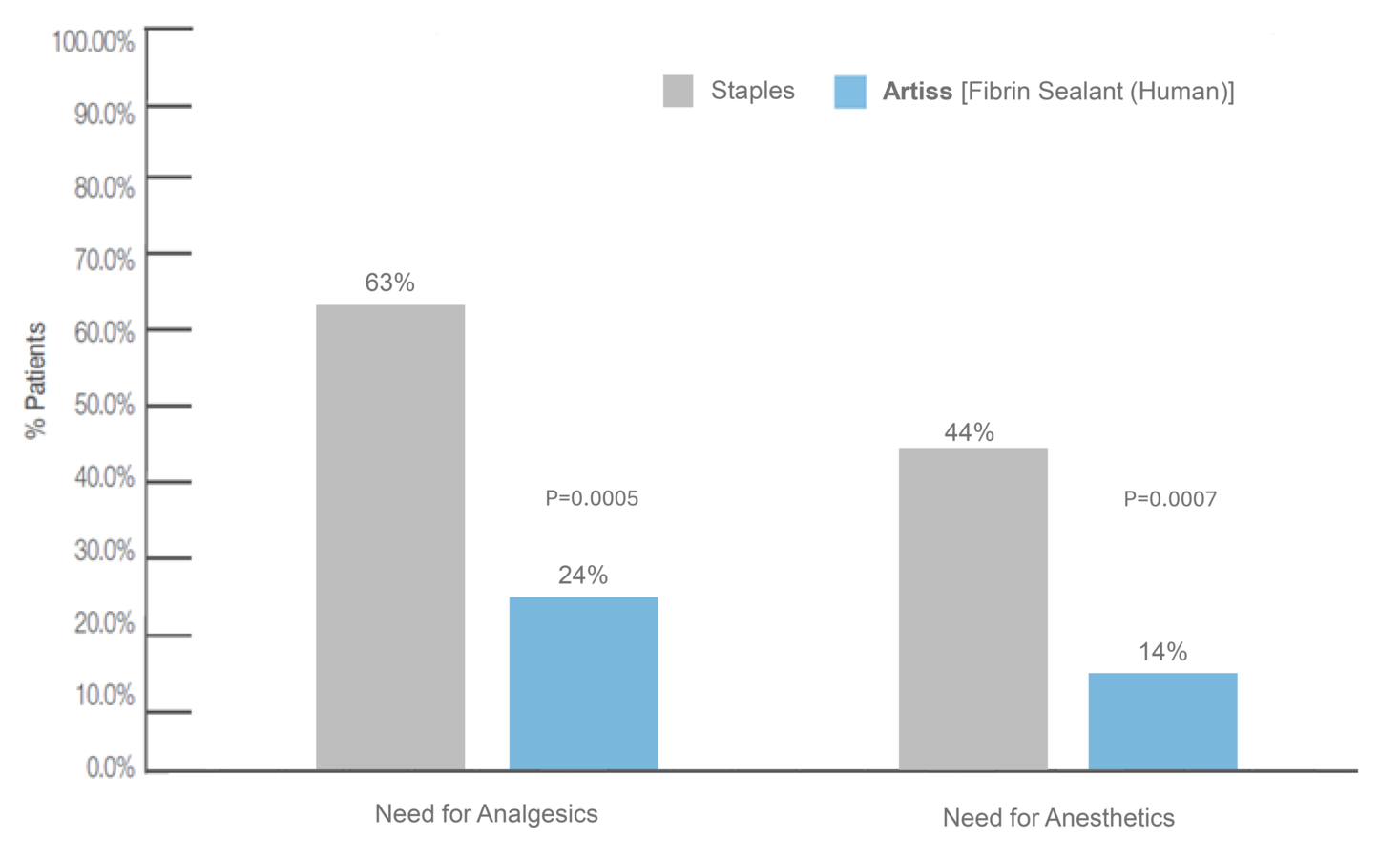
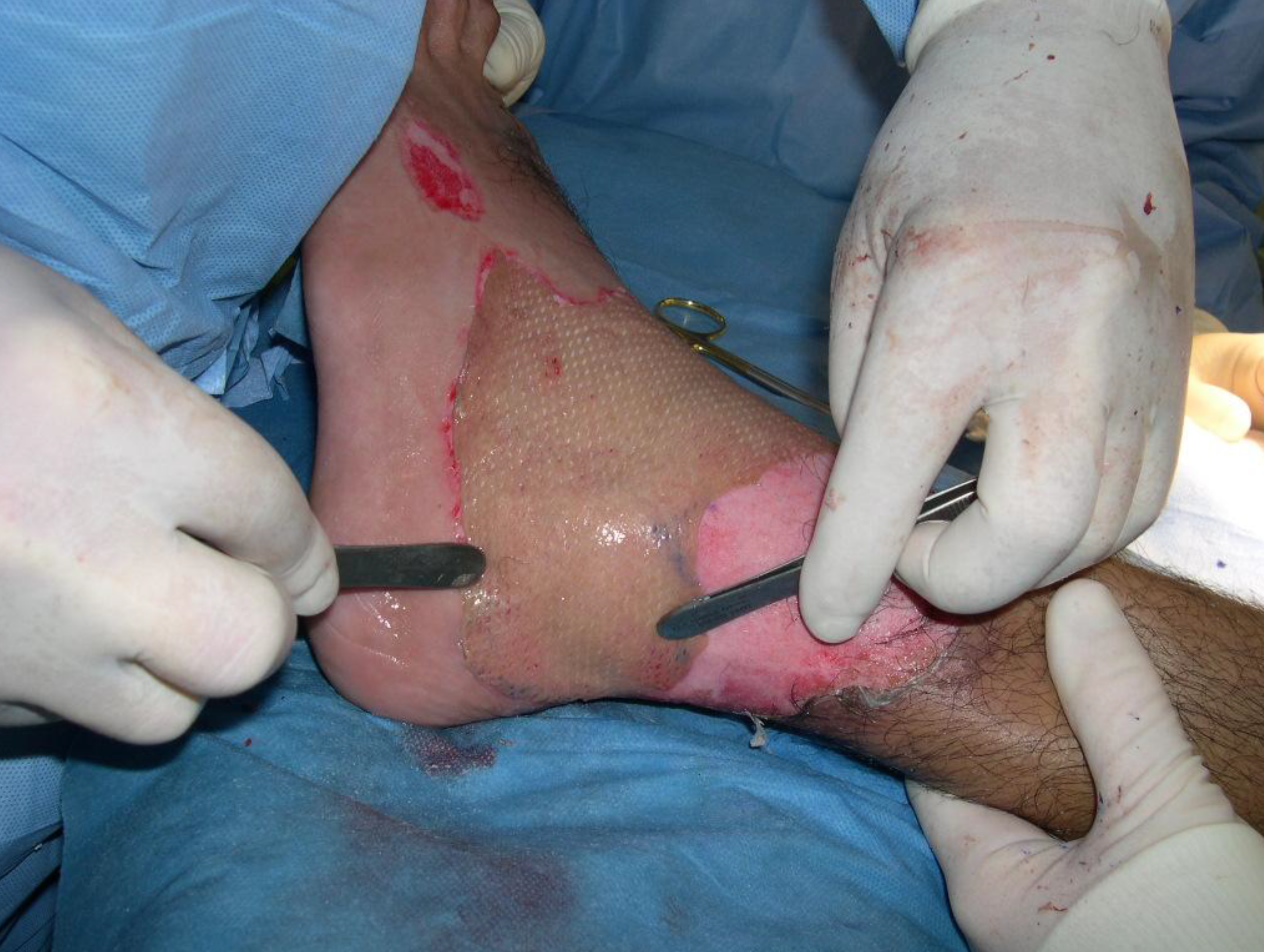
Sutures and staples have been used as the standard method of fixation.2 Staple removal can cause pain and anxiety in the patient and frequently requires intravenous analgesia and/or sedation, and in some instances conscious sedation or general anesthesia.4 The use of fibrin sealant has gained popularity as a graft fixation method because of the well-known adhesive properties.2 Because fibrin sealants are eventually broken down via the same mechanisms used in blood clot dissolution, they don't require additional healthcare visits for removal, making them an attractive alternative to staples or sutures.4
The only fibrin sealant currently indicated for skin graft fixation in burn surgery is Artiss [Fibrin Sealant (Human)] which has a slow-setting formulation to allow sufficient time for graft positioning.3,4 In 2018, we presented an abstract at the American Burn Association Annual Meeting describing an “enhanced recovery” burn surgery paradigm that utilized Artiss [Fibrin Sealant (Human)] fixation for 32 skin grafts with no (or minimal staples/sutures) without graft loss.5 We routinely use it in our practice now, and perform increasingly large and complex grafts without staples or sutures, even on functional, mobile areas such as hands.
Selected Risk Information: Artiss [Fibrin Sealant (Human)] is contraindicated in individuals with a known hypersensitivity to aprotinin and/or hypersensitivity to any of the active substances or excipients, including proteins such as fibrinogen, thrombin and human albumin.
Key Properties of ARTISS [Fibrin Sealant (Human)]
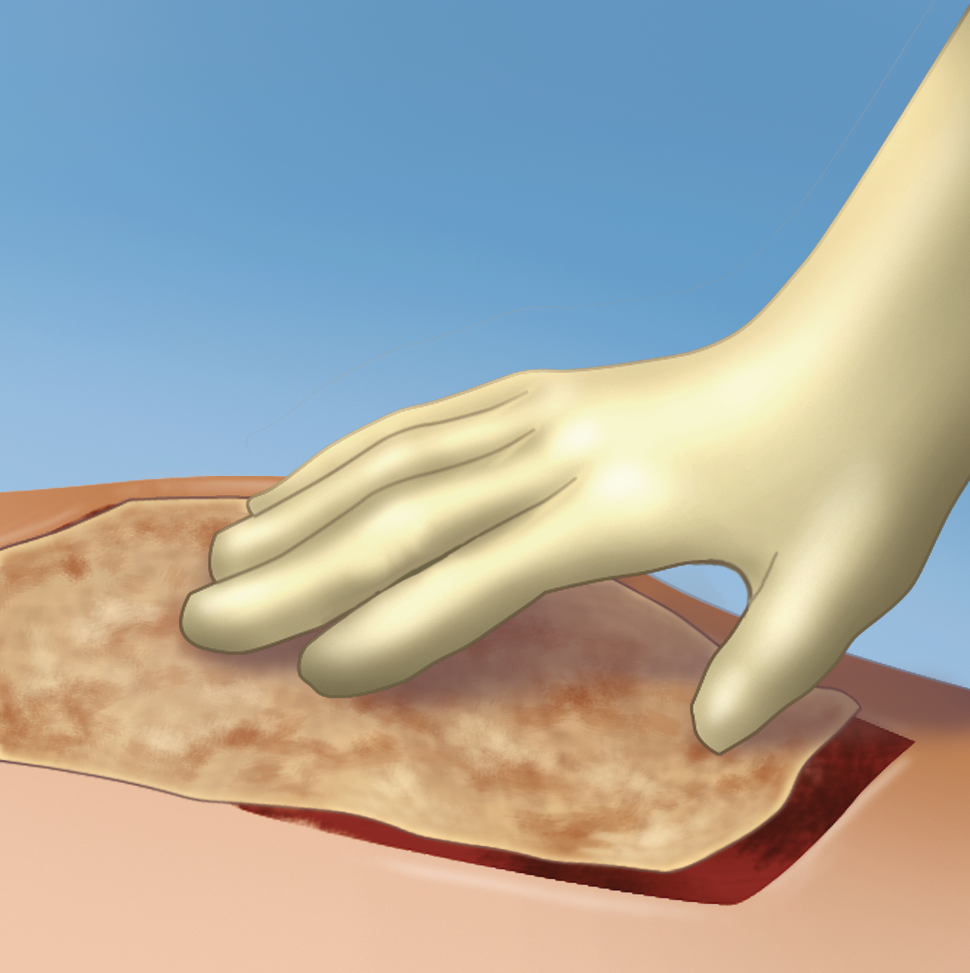
It provides full surface adherence of a skin graft to the wound bed.3,4
It decreases incidence of seroma/hematoma without requiring follow-up for staple/suture removal.3*
*Compared to standard of care
What is ARTISS [Fibrin Sealant (Human)]?
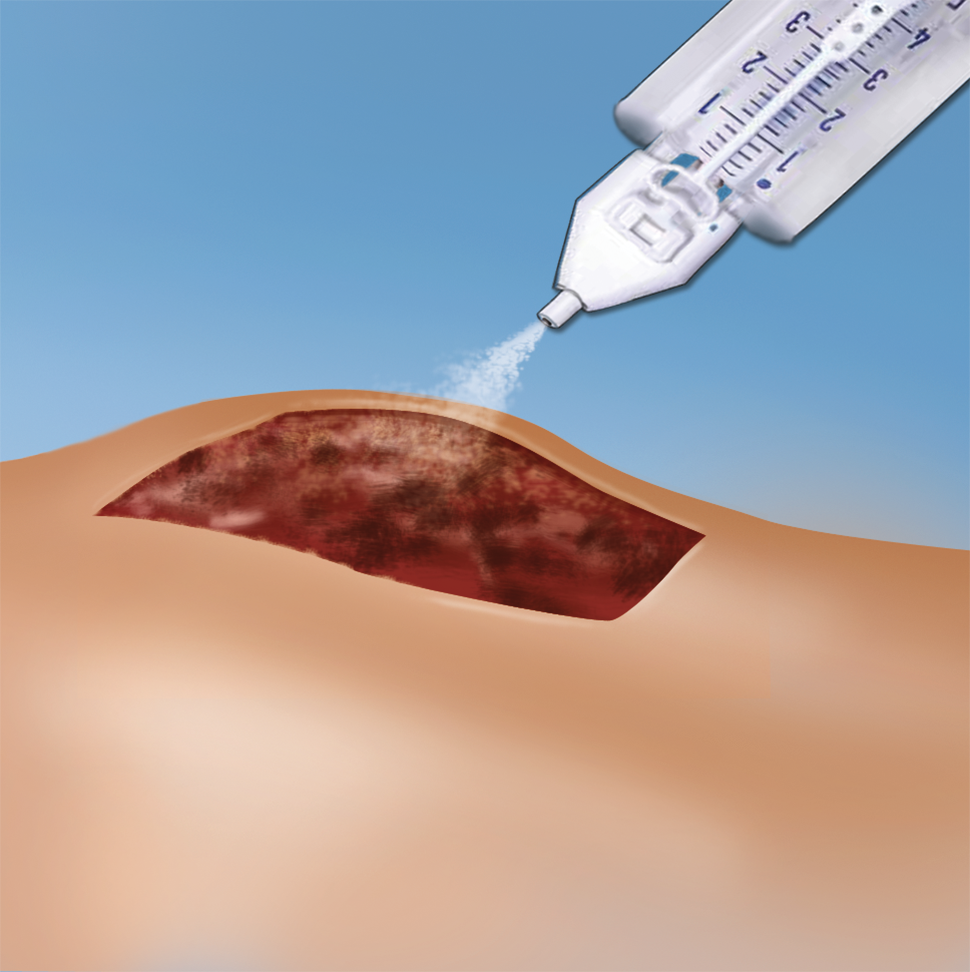
Artiss [Fibrin Sealant (Human)] received approval from the United States (US) Food and Drug Administration (FDA) in 2008.3 It consists of two plasma-derived components, a sealer protein solution and a thrombin solution.3 The sealer protein solution contains human fibrinogen 67-106 mg/mL, fibrinolysis inhibitor (synthetic) 2250-3750 KIU/mL and total protein 96-125 mg/mL.3 Human thrombin 2.5-6.5 IU/mL and calcium chloride 36-44 μmol/mL are components of the thrombin solution.3 Both compounds are provided frozen in two preloaded syringes presented in a single spraying device ready for topical application after thawing.3
What is the Indication?
Artiss [Fibrin Sealant (Human)] is indicated is indicated to adhere autologous skin grafts to surgically prepared wound beds resulting from burns in adult and pediatric populations greater than or equal to 1 year of age.3 It is also indicated to adhere tissue flaps during facial rhytidectomy surgery (face-lift).3 Artiss [Fibrin Sealant (Human)] is not indicated as an adjunct to hemostasis.3
How Does It Work?
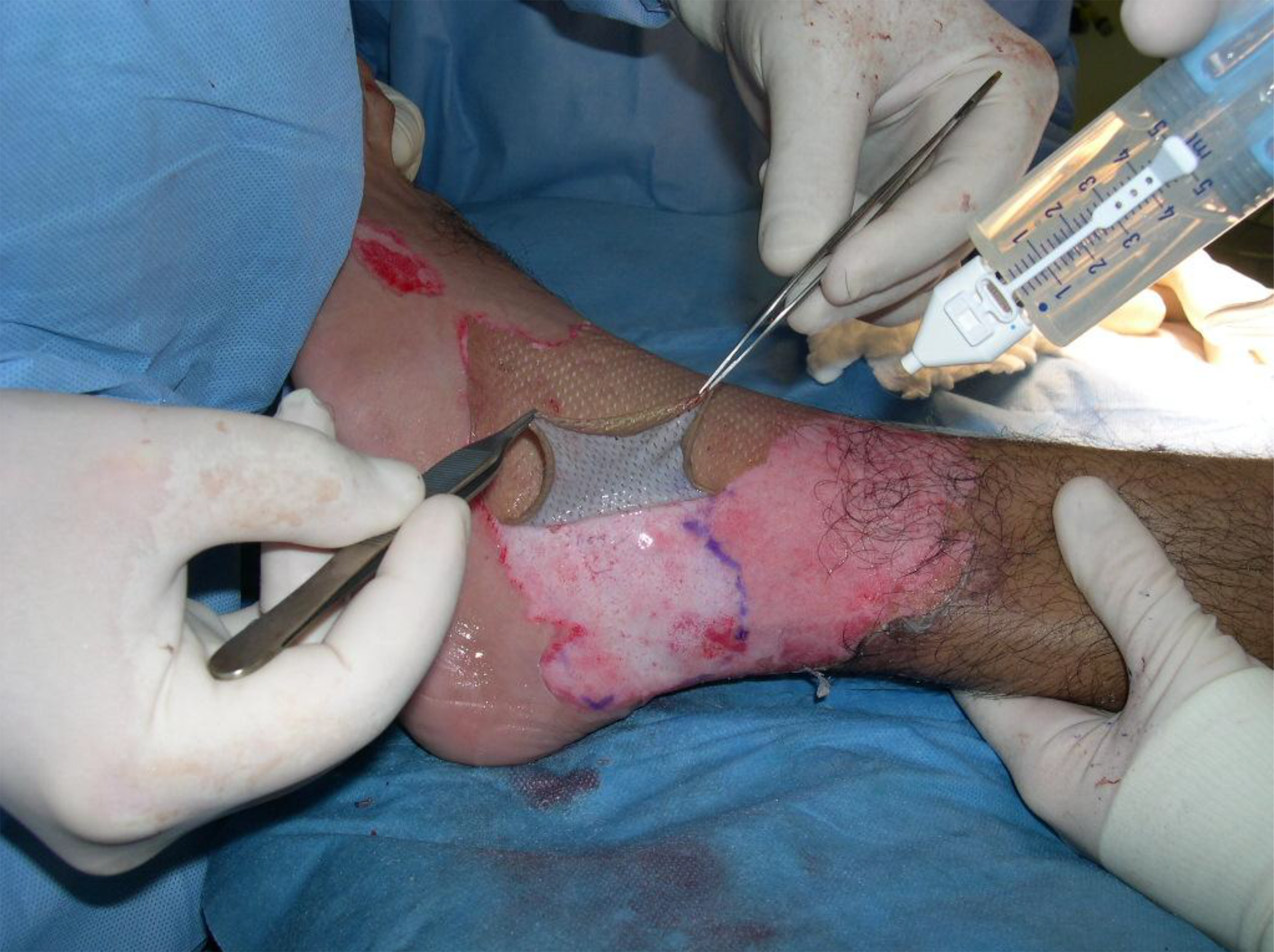
Upon application, the fibrinogen is transformed into a fibrin matrix that adheres to the wound surface and to the skin graft to be affixed (Video 1).3,4 The adhesive properties of Artiss [Fibrin Sealant (Human)] provide full surface adherence of the graft to the wound bed, minimizing the dead space that exists when grafts are attached using point fixation techniques such as staples.4 It decreases the incidence of seroma and hematoma, and full surface adherence minimizes areas of dead space between the wound bed and applied tissues.3