Five Advantages of Using Hemostatic and Adhesion Prevention Agents in Surgery
FIVE ADVANTAGES OF USING HEMOSTATIC AND ADHESION PREVENTION AGENTS IN SURGERY
In the high-stakes world of surgery, achieving precise control over bleeding is critical. Despite advances in surgical technique, excess bleeding remains a major complication associated with surgery and contributes to poor clinical outcomes.1 In addition, the larger the residual amount of blood and serosanguinous fluid, the more frequently adhesions can occur.2 Thus, the management of bleeding plays a crucial role in optimizing surgical outcomes. The development of hemostatic and adhesion prevention agents has provided surgeons with powerful tools that facilitate rapid hemostasis, mitigate intraoperative blood loss and reduce the potential for adhesions and subsequent complications.1,3
This article aims to spotlight the benefits that hemostatic and adhesion prevention agents bring to the surgical table. We will look at the top five advantages of employing these important tools in surgical procedures. Whether you are already familiar with these agents or simply eager to advance your knowledge on the latest advancements in surgery, this article is designed to enrich your understanding and clinical practice. Dr. Calvin Holloway, Medical Science Liaison at Baxter Healthcare, will discuss these agents’ roles in patient safety, surgical efficiency, and surgical outcomes.

Introduction
Hemostasis, a cornerstone of surgical practice, involves a multifaceted coagulation cascade from the initial vasoconstriction after injury to the final formation of a stable fibrin clot.4 Inadequate surgical hemostasis may lead to transfusion and/or other bleeding-related complications, and residual bleeding can predispose patients to the formation of postoperative adhesions and other complications.2,5 Approximately 30% of patients will experience bleeding-related complications during surgery that impact recovery.5 This correlation between meticulous hemostasis and risk for adhesion formation underscores the important roles that surgical technique and the strategic use of hemostatic agents and adhesion barriers play in postoperative outcomes.2,6
What Are Hemostatic and Adhesion Prevention Agents?
Bleeding and Adjunctive Hemostatic Agents
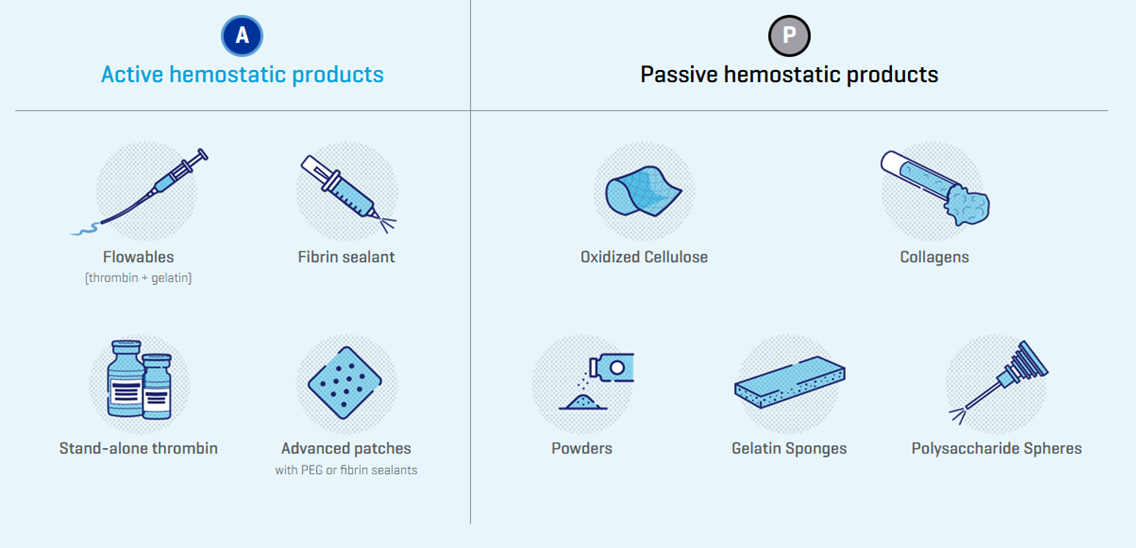
Effective hemostasis as part of a blood management strategy is a key requirement for patients undergoing surgery.7 The primary methods to achieve surgical hemostasis include sutures, clips, and a wide variety of electrosurgical devices.7 Topical hemostatic products are used as adjuncts to established primary methods to facilitate hemostasis.7 They are used across a wide variety of surgeries and grades of bleeding, highlighting their safe and effective use in clinical practice.7 These hemostatic agents can be further categorized as either ‘passive’ or ‘active’ products based on their mechanism of action.7 Passive products provide a physical structure to mechanically facilitate the aggregation of platelets so that a clot can form while ‘active’ products deliver their mechanism of action on the clotting cascade in a biologically active manner (Figure 1).7
Figure 1. Active and Passive Hemostatic Agents7
Abdominopelvic Adhesions and Adhesion Barriers
Abdominopelvic adhesions are abnormal fibrous bands that connect surfaces or organs within the peritoneal cavity that are normally separated.8,9,10 Surgical adhesions result from the pathologic healing response of the peritoneum upon injury (most commonly surgical trauma) and may lead to postoperative complications such as pain, bowel obstruction and infertility in addition to adding complexity and difficulty to re-operative procedures.8,10,11 Several key strategies exist for reducing the formation of adhesions including minimization of surgical trauma through improved surgical technique, use of minimally invasive approaches, meticulous attention to operative techniques, delicate purposeful tissue handling and uncompromising hemostasis.10,12
In addition, there are commercially available adjunct products such as Seprafilm Adhesion Barrier (hyaluronate carboxymethylcellulose [HA-CMC]) that act to minimize the formation of surgical adhesions by mechanical separation of injured tissues surfaces during the crucial period of healing after surgery (Video 1).13,14 These commercially available adhesion barriers may come in the form of solid membranes, gels and/or liquids.10
Video 1. Reduce Adhesions, Protect Your Patients' Future
Commonly used types of adhesion barriers in the United States10:
- Seprafilm Adhesion Barrier (HA-CMC): Published studies in both general surgery and gynecological procedures have shown HA-CMC adhesion barriers reduce adhesion formation and the risk of reoperation for adhesive small bowel obstruction (relative risk 0.49, 95% CI 0.28–0.88).
- Interceed Adhesion Barrier (oxidized regenerated cellulose): Only studied in gynecological procedures. Reduces incidence of adhesion formation (relative risk 0.51, 95% CI 0.31–0.86).
- Adept Adhesion Reduction Solution (4% icodextrin): A liquid-based adhesion barrier with an established safety profile in gynecological surgery. The liquid formulation aids application in laparoscopic surgery. Reduces recurrence of adhesive small bowel obstruction following surgery in one trial (relative risk 0.20, 95% CI 0.04–0.88).
Five Advantages of Hemostatic and Adhesion Prevention Agents
Advantage 1: Rapid Intraoperative Hemostasis
Hemorrhage is the leading cause of potentially preventable mortality and the effective and rapid management of bleeding during surgical procedures is essential for promoting positive patient outcomes including shorter operating times, reduced wound exposure, decreased transfusion requirements, and improved patient recovery times.5,15,16 Thus, there is no substitute for prompt surgical control of the source of hemorrhage.15 In appropriate cases, adjunctive topical hemostatic agents, such as Floseal Hemostatic Matrix, may be useful in efficiently managing persistent bleeding (Video 2).15–17
Video 2. FLOSEAL Matrix: Rapid Hemostasis Case Video
Advantage 2: Reduction of Intraoperative Blood Loss
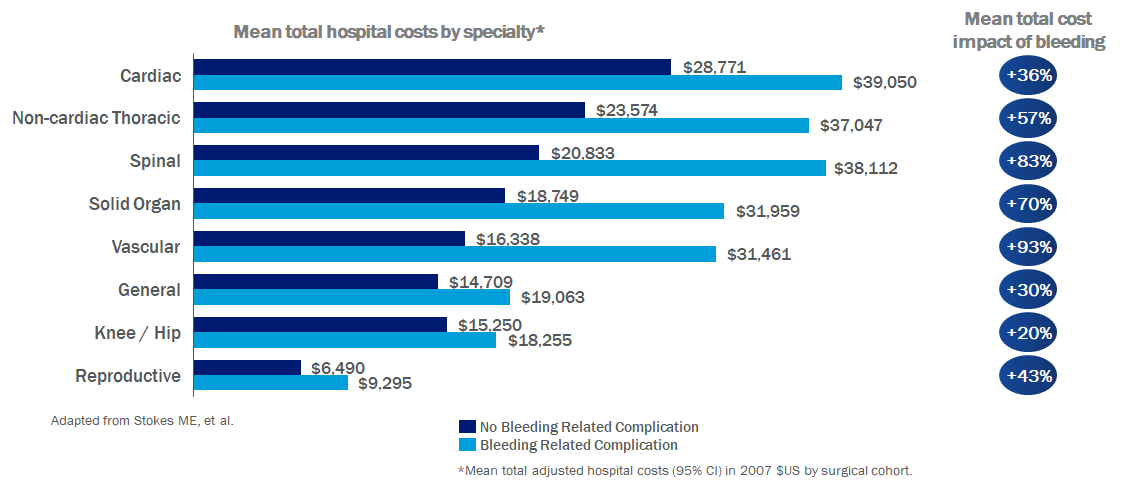
Hemostatic agents and tissue sealants are routinely used to mitigate and prevent blood loss during surgical repair.18 By promoting rapid blood clotting, both passive and active hemostatic agents offer improved blood conservation by reducing blood loss.19 As a result of intraoperative blood loss, the need for allogenic or autologous blood transfusions and the risks associated with blood transfusions are increased.19 Reduced length of stay in the intensive care unit (ICU) and overall length of hospital stay have been related to reductions in the amount of blood transfused.19 Moreover, bleeding-related complications significantly impact hospital costs resulting in incremental differences in mean total costs (bleeding-related complication versus no complication) of up to $17,279 per surgical procedure (Figure 2).*5
*based on a 2006-2007 retrospective analysis using Premier's PerspectiveTM hospital database
Figure 2. Increased Costs Due to Bleeding Complications5
Advantage 3: Versatility in Various Surgical Procedures
Hemostatic agents available to surgeons today have extensive evidence of effective and safe use across a wide variety of surgical procedures including cardiothoracic, general (e.g., bariatric, colorectal, hepatic), gynecologic, orthopedic, oncologic, plastic, trauma, urologic and vascular surgery.7 These agents may be used in minor and major operations and their ability to be applied in numerous surgical contexts make them invaluable tools for surgeons.7
As no evidence-based practice guidelines are currently available to facilitate clinically informed decisions on approaches to hemostasis, certain tools or processes have been developed to help guide surgeons on selecting the appropriate hemostatic agent based on critical factors such as bleeding severity, bleeding risk, and surgery type.7 One of these tools is known as the Validated Intraoperative Bleeding Scale (‘The VIBe Scale tool’). The VIBe Scale tool is the first surgeon-validated bleeding scale to aid in the evaluation of bleeding severity in open surgical procedures across surgical specialties (Figure 3).20 The VIBe Scale tool is graded on a 5-point scale from 0 (no bleeding) to 4 (life threatening) and provides a standardized, reproducible method for assessing bleeding severity to ultimately help guide surgical teams on appropriate hemostatic product choice.20 For example, surgeons should consider an active hemostatic agent as the first approach for higher grades of bleeding.7
Figure 3. VIBe Scale Tool for Grading Bleeding Severity20*
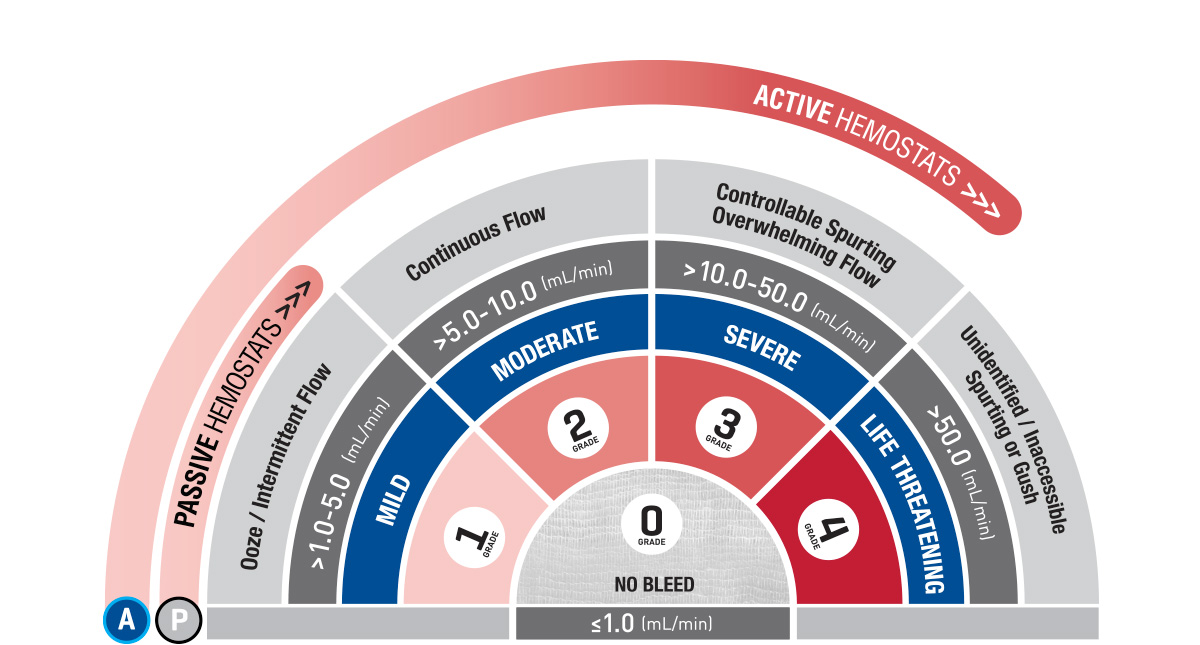
Grade 0: No Bleeding
- Superficial Nephric Abrasion
Grade 1: Mild
- Superficial Hepatic Abrasion
Grade 2: Moderate
- Superficial Cardiac Laceration
Grade 3: Severe
- Partial Hepatectomy
Grade 4: Life Threatening
- Supra-Renal Abdominal Aortotomy

Advantage 4: Improved Surgical Efficiency
Rapid and effective hemostasis allows the surgeon to retain visualization of the surgical field.19 This can both reduce the procedure time and the risk of accidental injury.19 In turn, a reduction in surgery time may lead to potential savings in operating room costs.19 Effective hemostasis should also decrease the morbidity and mortality associated with surgical procedures.19 Surgeons can focus more on the critical aspects of the operation without the interruptions caused by uncontrolled bleeding.
Similarly, use of adhesion prevention agents may also facilitate improved outcomes in subsequent surgeries.22 Mounting evidence demonstrates that adhesions are now the most frequent complication of abdominopelvic surgery, particularly in subsequent surgeries or reoperations which comprise as much as 40-70% of elective general surgery procedures.22,23 Managing patients with severe adhesions is problematic posing a tremendous burden to the patient and surgeon.22 Adhesions can turn a simple procedure into a complex one riddled with potential pitfalls that can have serious consequences for the patient.22 Despite this, many surgeons are still not aware of the extent of the problem or the potentially serious consequences.22 Although contemporary surgery has shifted towards minimally invasive techniques, minimally invasive surgery alone does not fully alleviate the burden of adhesion-related complications.23 Recently, adhesion barriers have demonstrated the potential to reduce adhesion formation and the incidence of subsequent complications.23 Therefore, the use of adhesion barriers in addition to meticulous surgical technique may be crucial in avoiding these serious sequelae.22
Advantage 5: Reduced Risk of Postoperative Complications
Up to 93% of patients undergoing abdominal operations develop adhesions, which can lead to complications like pain, bowel obstruction and infertility which ultimately translate to increased hospital costs.2,8,24,25
Considerations for adhesion prevention should be based upon an understanding of peritoneal repair and what goes awry when adhesions develop.26 Adhesion formation is thought to be the result of efforts by the body to restore the supply of oxygen and nutrients to tissue that has been injured during surgery.26 This surgically-induced tissue hypoxia produces an inflammatory-like response and the generation of reactive oxygen- and nitrogen species that invoke the release of histamine, cytokines, and growth factors.26 As a result, collections of blood and serosanguinous tissue exudates on the surface of the injured tissues ultimately form a fibrinous mass.26 This process persists during and beyond the 3 to 5 days required for remesotheliazation.26 Thus, the critical time period to prevent or diminish adhesion development occurs within the initial 3 to 5 days after the surgical procedure.26 This emphasizes the critical role that surgical technique, meticulous hemostasis and strategic use of hemostatic agents and adhesion barriers play in reducing adhesion development and enhancing postoperative outcomes.2,6
Up to 93% of Patients Develop Adhesions Following Abdominal Operations2
Increased Risk of Complications
~30% 10 year readmission rate of abdominal surgical patients who may return for adhesion-related complications such as pain, bowel obstruction and infertility.2,25
Increased Hospital Costs
$2.3B is the estimated annual economic impact of adhesiolysis procedures.24
Clinical Data:
In the pivotal study for Seprafilm Adhesion Barrier, 183 patients with ulcerative colitis or familial polyposis undergoing colectomy with ileal pouch anal anastomosis and temporary loop ileostomy were enrolled.5 The incidence, extent and severity of adhesions to the midline incision were evaluated at the time of ileostomy closure with or without the use of the adhesion barrier.5 Forty-three of 85 patients (51%) in the intervention group were free of adhesions 8–12 weeks after the procedure versus 6% (5/90) of those who underwent surgery without the adhesion barrier (p<0.00000000001).5 Dense adhesions were reported in only 13 patients (15%) who received the adhesion barrier compared with 52 patients (58%) in the control group (p<0.0001).5
In a pivotal study for Adept Adhesion Reduction Solution (4% icodextrin liquid adhesion barrier), the objective was to evaluate the safety and efficacy in adhesion reduction as compared to lactated Ringer’s solution (LRS) after laparoscopic gynecologic surgery with adhesiolysis.27 Adhesion scoring was performed at time of operation and at follow-up laparoscopy 4-8 weeks post-surgery, assessing the presence, extent, and severity of adhesions, with clinical success defined as a reduction in the number of adhesions of at least 3 or 30% of sites lysed (whichever is greater).27 Results demonstrated a statistically significant reduction in adhesion formation with Adept Adhesion Reduction Solution as compared with LRS, with 49% of the patients receiving Adept Adhesion Reduction Solution achieving clinical success versus 38% in the LRS group (p=0.018).27
A Look to the Future
The field of surgery continues to evolve as advancement in science and technology drive medical innovation geared toward enhancing patient care, improving clinical outcomes and shortening recovery times. Hemostatic and adhesion prevention agents are a crucial subset of these advancements that help create safer surgeries while minimizing complications.28 The emerging realization and understanding of their importance in surgery will undoubtedly have a positive impact on both the clinical and economic challenges facing healthcare today.28
Summary
The development of adjunctive hemostatic and adhesion prevention agents represent significant advancements in surgical technology and clinical practice. As their benefit to patient safety, surgical efficiency and clinical outcomes becomes more apparent over time, these agents will be invaluable tools to surgeons of today and tomorrow alike.
SEPRAFILM ADHESION BARRIER INDICATIONS AND IMPORTANT SAFETY INFORMATION
INDICATIONS
Seprafilm Adhesion Barrier is indicated for use in patients undergoing abdominal or pelvic laparotomy as an adjunct intended to reduce the incidence, extent, and severity of postoperative adhesions between the abdominal wall and the under-lying viscera such as omentum, small bowel, bladder, and stomach, and between the uterus and surrounding structures such as tubes and ovaries, large bowel, and bladder.
IMPORTANT RISK INFORMATION
Seprafilm Adhesion Barrier is contraindicated in patients with a history of hypersensitivity to Seprafilm and/or to any component of Seprafilm.
Seprafilm Adhesion Barrier is contraindicated for use wrapped directly around a fresh anastomotic suture or staple line; as such use increases the risk of anastomotic leak and related events (fistula, abscess, leak, sepsis, peritonitis).
Seprafilm Adhesion Barrier must be used according to the instructions for use. Seprafilm Adhesion Barrier is supplied sterile and must not be re-sterilized. Seprafilm Adhesion Barrier is for single use only. Every opened and unused Seprafilm pouch must be discarded. Do not use product if pouch is damaged or opened.
The number of sheets used should be just adequate to cover the under surface of the abdominal wall or uterine incision in a single layer.
In patients who have ovarian, primary peritoneal or fallopian tube malignancies, Seprafilm Adhesion Barrier use has been reported to have an increased risk of intra-abdominal fluid collection and/or abscess, particularly when extensive debulking surgery was required.
The safety and effectiveness of Seprafilm Adhesion Barrier has not been evaluated in clinical studies for the following: Patients with frank infections in the abdominopelvic cavity; patients with abdominopelvic malignancy, such as device use on resected liver surfaces following hepatectomy for malignant liver tumors; device placement in locations other than directly beneath an abdominal wall incision following laparotomy, or directly on the uterus following open myomectomy (not laparoscopic); patients with ongoing local and/or systemic inflammatory cell responses; device use in the presence of other implants, e.g. surgical mesh; patients requiring re-operation within four weeks of Seprafilm Adhesion Barrier placement – during anticipated time of peak adhesion formation. Foreign body reactions have occurred with Seprafilm Adhesion Barrier.
The safety and effectiveness of Seprafilm Adhesion Barrier in combination with other adhesion prevention products and/or in other surgical procedures not within the abdominopelvic cavity have not been established in clinical studies.
The safe and effective use of Seprafilm Adhesion Barrier in pregnancy and Cesarean section has not been evaluated. No clinical studies have been conducted in pregnant women or women who have become pregnant within the first month after exposure to Seprafilm Adhesion Barrier. Therefore, this product is not recommended for use during pregnancy and avoidance of conception should be considered during the first complete menstrual cycle after use of Seprafilm Adhesion Barrier.
Long term clinical outcomes such as chronic pain and infertility have not been determined in clinical studies.
Rx Only. For safe and proper use of this device refer to the complete Instructions for Use.
ADEPT Adhesion Reduction Solution [4% Icodextrin] Indications
Adept Adhesion Reduction Solution is indicated for use intraperitoneally as an adjunct to good surgical technique for the reduction of post-surgical adhesions in patients undergoing gynecological laparoscopic adhesiolysis.
Important Risk Information for ADEPT
Adept Solution is for direct intraperitoneal administration only. NOT for intravenous (IV) administration.
Adept is contraindicated in patients with known or suspected allergy to cornstarch based polymers e.g. icodextrin, or with maltose or isomaltose intolerance, or with glycogen storage disease.
Adept is contraindicated in laparotomy, in cases involving bowel resection or repair, or appendectomy and in surgical cases with frank abdomino-pelvic infection.
There have been rare reports of sterile peritonitis following the use of icodextrin.
Leakage of Adept from port sites may lead to wound healing complications; meticulous fascial closure may reduce leakage through laparoscopic port sites post-operatively.
There have been rare reports of hypersensitivity reactions, pulmonary edema, pulmonary effusion and arrhythmia.
Anaphylaxis has been reported in a few patients.
Maltose metabolites of icodextrin may interfere with blood glucose measurement in diabetic patients who use rapid blood glucose systems that are not glucose specific.
In the pivotal study, the most frequently occurring treatment related adverse events between surgeries were post procedural leaking from port sites, labial, vulvar or vaginal swelling and abdominal distention.
Rx Only: For safe and proper use of this device, please refer to full Instructions For Use.
FLOSEAL HEMOSTATIC MATRIX INDICATIONS AND IMPORTANT RISK INFORMATION
INDICATIONS
Floseal Matrix is indicated in surgical procedures (other than ophthalmic) as an adjunct to hemostasis when control of bleeding by ligature or conventional procedures is ineffective or impractical.
Important Risk Information
Do not inject intravascularly.
Do not inject or compress Floseal Matrix into blood vessels.
Do not apply Floseal Matrix in the absence of active blood flow, e.g., while the vessel is clamped or bypassed, as extensive intravascular clotting and even death may result.
Do not administer to patients with a history of hypersensitivity to Recothrom Thrombin Topical (Recombinant), any components of Recothrom, or hamster proteins.
Do not use Floseal Matrix in patients with known allergies to materials of bovine origin.
Do not use Floseal Matrix in the closure of skin incisions because it may interfere with the healing of the skin edges due to mechanical interposition of gelatin.
Floseal Matrix is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis. Floseal Matrix is not intended to be used as a prophylactic hemostatic agent.
As with other hemostatic agents, do not apply Floseal Matrix to sites where there is negative peripheral venous pressure, as material may be drawn into the vascular system potentially resulting in life threatening thromboembolic events.
Excess Floseal Matrix (material not incorporated in the hemostatic clot) should always be removed by gentle irrigation and suctioned out of the wound.
The particles of Floseal Matrix swell approximately 10-20% (upon contact with blood or other fluids) and surgeons should consider its potential effect on the surrounding anatomic areas. Maximum swell volume is achieved within about 10 minutes.
Floseal Matrix should not be used in the presence of infection. Floseal Matrix should be used with caution in contaminated areas of the body.
The safety and effectiveness of Floseal Matrix for use in ophthalmic procedures has not been established.
Floseal Matrix should not be used for controlling post-partum bleeding or menorrhagia.
The safety and effectiveness of Floseal Matrix has not been established in children under 2 years of age and pregnant women.
It is not known whether Floseal Matrix can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Floseal Matrix should be administered to a pregnant woman only if clearly needed.
Do not use air to remove residual Floseal Matrix from Applicator tip. The Applicator tips should not be cut, as tissue injury from sharp edges may result.
Hypersensitivity reactions, including anaphylaxis, may occur. Recothrom thrombin is produced in a genetically modified Chinese Hamster Ovary (CHO) cell line and may contain hamster or snake proteins.
For single use only. Do not re-sterilize.
Floseal Matrix should not be applied before the application site is cleaned to remove any antiseptics that may contain alcohol, iodine, or heavy metal ions.
When placed into cavities or closed tissue spaces, gentle approximation is advised. Do not compress.
As with other hemostatic agents, do not aspirate Floseal Matrix into extracorporeal cardiopulmonary bypass circuits or autologous blood salvage circuits.
Do not use Floseal Matrix on bone surfaces where adhesives, such as methylmethacrylate or other acrylic adhesives, will be required to attach a prosthetic device.
Floseal Matrix should not be used for the primary treatment of coagulation disorders.
The safety and effectiveness of the combined use of Floseal Matrix with antibiotic solutions or powders has not been established.
The safety and effectiveness for use in neurosurgical and urological procedures has not been established through randomized clinical studies.
In urological procedures, Floseal Matrix should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
Antibody formation to Recothrom thrombin occurred in <1% of patients. None of the antibodies detected neutralized native human thrombin.
Thrombin must be added to the Gelatin Matrix prior to use.
Rx Only. For safe and proper use of this device, refer to the full Instructions for Use.
Baxter, Seprafilm, Adept, Floseal, Recothrom, and VIBe Scale are registered trademarks of Baxter International Inc., or its subsidiaries.
Interceed is a registered trademark of Ethicon Inc.