TISSEEL
*Sealing indicated to prevent leakage from colonic anastomoses following the reversal of temporary colostomies.
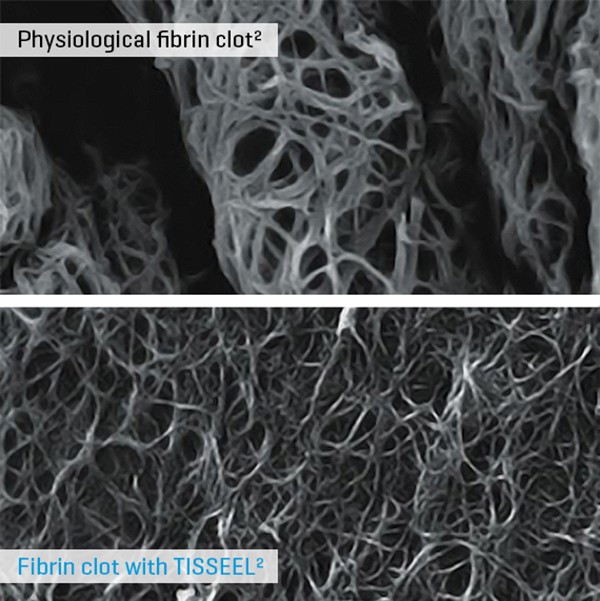
Mimics Physiological Clot
Only Tisseel contains synthetic aprotinin, which is a highly effective clot stabilizer that prevents premature clot degradation.1
Selected Risk Information: Do not use TISSEEL in individuals with a known hypersensitivity for aprotinin. Please see important risk information for additional details.
Introducing Gasless Spray Tip
Ready when you are, the new applicator technology
- Saves Time- Eliminates the need for external gas sources,3 saving valuable OR setup time
- Dual functionality- Gasless Spray Tip delivers your choice of broad spray or precise drip3
- Open or Laparoscopic- Your choice of Open, 25 cm MIS and 40 cm MIS spray applicators
- Quick Connect- Secure Snap Lock attachment to PRIMA syringe3
Mechanism of Action
Human sealer protein and human thrombin transform fibrinogen into fibrin, mimicking the final step of the coagulation cascade to form a clot.1
Additional Product Benefits
Wide Range of Applicators
TISSEEL offers a complete portfolio of drip and spray applicators, allowing surgeons to select the right tools to address individual patient needs.1
Precise Placement
The DUPLOSPRAY System allows for bleeding to be targeted laparoscopically by getting as close as 2-5 cm to the tissue.¹
Do not spray TISSEEL where the recommended minimum distance from the applicator tip to the target site cannot be assured. Please see full Prescribing Information for recommended distances and pressure.
Indications and Important Risk Information
TISSEEL [Fibrin Sealant] Indications
Hemostasis: TISSEEL is a fibrin sealant indicated for use as an adjunct to hemostasis in adult and pediatric patients (> 1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. TISSEEL is effective in heparinized patients.
Sealing: TISSEEL is a fibrin sealant indicated as an adjunct to standard surgical techniques (such as suture and ligature) to prevent leakage from colonic anastomoses following the reversal of temporary colostomies.
Important Risk Information for TISSEEL [Fibrin Sealant]
For Topical Use Only. Do not inject TISSEEL directly into the circulatory system or into highly vascularized tissue. Intravascular application of TISSEEL can lead to intravascular coagulation, can result in life- threatening thromboembolic events, and can increase the likelihood and severity of acute hypersensitivity reactions in susceptible patients. To minimize the risk of intravascular application, exercise caution when using TISSEEL in surgery.
Do not use TISSEEL in individuals with a known hypersensitivity to aprotinin.
Do not use TISSEEL for treatment of severe or brisk arterial or venous bleeding. In these situations, TISSEEL will be washed away in the flow of blood before hemostasis can be attained.
Do not spray TISSEEL where the minimum recommended distance from the applicator tip to the target site cannot be assured.
Hypersensitivity or allergic/anaphylactoid reactions can occur with the use of TISSEEL. Such reactions may especially be seen if TISSEEL is applied repeatedly over time or in the same setting, or if systemic aprotinin has been administered previously.
Aprotinin is known to be associated with anaphylactic reactions. Even in the case of strict local application of aprotinin, there is a risk of anaphylactic reactions to aprotinin, particularly in the case of previous exposure.
Discontinue administration of TISSEEL in the event of hypersensitivity reactions. Remove remaining product from the application site.
Air or gas embolism has occurred when fibrin sealant was administered using pressurized gas. This can occur if a spray device is used at higher than recommended pressures and in closer than recommended proximity to the tissue surface.
When using the EASYSPRAY device, or an equivalent spray device for open surgical procedures cleared by FDA, TISSEEL must not be sprayed in enclosed body areas and must be sprayed onto only visible application sites.
TISSEEL is denatured when exposing to solutions containing alcohol, iodine or heavy metals. If any of these substances have been used to clean the wound area, the area must be thoroughly rinsed before the application of TISSEEL.
Apply TISSEEL as a thin layer by dripping or spraying using cannula or spray set. Excess clot thickness can negatively interfere with wound healing.
The safety and effectiveness of TISSEEL used alone or in combination with biocompatible carriers in neurosurgical procedures or other surgeries involving confined spaces have not been evaluated; its use in this setting is not FDA approved.
TISSEEL is made from human plasma. It may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Please see accompanying full Prescribing Information
Gasless Tip Applicator Instructions for Use