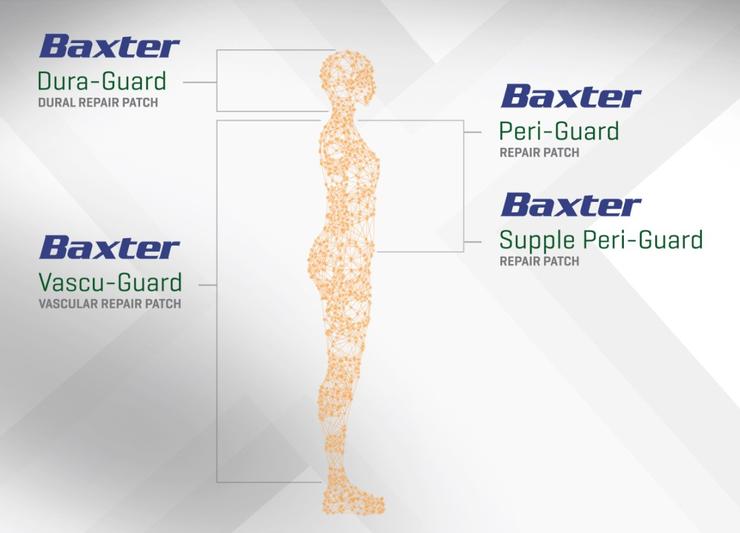
TISSUE GUARD FAMILY SURGICAL PATCHES
See Indications
35+ YEARS OF CLINICAL HISTORY
With over 30 years13 of global clinical history, our Tissue Guard family of surgical patches have supported overall patient healing outside of the OR through low inflammatory response,3 reduced risk of CSF leaks through watertight closure,13,14 and high biocompatibility, low antigenicity and tissue integration with reliable suture retention, resistant to suture line bleeding.4-10 The patches are recognized for providing strength, durability, and superior handling in soft tissue repair.2
Native collagen grafts derived from bovine pericardium, TISSUE GUARD Family of Surgical Patches offer surgeons a strong, suturable implant with excellent handling characteristics similar to autologous tissue4
Strength
Strong yet supple material with consistency similar to autologous tissue2,4,10
Handling
Easy to manipulate, contour and suture to the anatomy2,3
Biocompatible
Inert, low immunogenic properties with excellent host tissue response2,4,7
Resistant
Reapproximates to suture, resisting leakage and minimal suture line bleeding3,4,10,11
DURA-GUARD Dural Repair Patch
For use as a dura substitute for the closure of dura during neurosurgery
APPLICABLE PROCEDURES:
Tumor Resection
Trauma
Congenital Disorders
CLINICAL BENEFITS:
Strong yet flexible material with minimal elasticity similar to native dura12,26
Easy to manipulate, contour and suture to the anatomy3
Reduces risk of CSF leaks through watertight closure13,14
Good for high and low pressure CSF sites12*
*Preclinical data. Results may not correlate to results in humans.
VASCU-GUARD Vascular Repair Patch
For use in peripheral vascular reconstruction, including the carotid, renal, iliac, femoral, profunda, tibial vessels and arteriovenous access revisions.

APPLICABLE PROCEDURES
Carotid endarterectomy
Profundaplasty
Femoral, Iliac, Renal, and Tibial patching
CLINICAL BENEFITS
Resists suture line bleeding2,4
Reduced long-term restenosis rates when compared to Dacron angioplasty and primary closure15
Facilities Doppler ultrasound directly through patch15
Low incidence of post-operative and long term adverse events10, 15, 16, 28
PERI-GUARD & SUPPLE PERI-GUARD Repair Patch
For use as a prosthesis for surgical repair of pericardial structures and soft tissue deficiencies which include:
defects of the abdominal and thoracic wall
hernias (diaphragmatic, femoral, incisional, inguinal, lumbar, scrotal and umbilical)
PERI-GUARD is also intended for use as a patch for intracardiac defects, great vessel, septal defects and annulus repair, and suture-line buttressing.

APPLICABLE PROCEDURES:
Repair of Pericardial structures
Intracardiac & great vessel & Septal defect repair
Annulus & Soft tissue deficiency repairs
Suture-line buttressing
Thoracic & cardiothoracic surgery
CLINICAL BENEFITS:
No long-term signs of graft degeneration following aortic value reconstruction21
Favorable mid to long-term outcomes following aortic value reconstruction21,22
Complete lung expansion following chest wall reconstruction23
No signs of herniation post chest wall reconstruction24
Reduced adhesions and inflammation24,25
Additional TISSUE GUARD Family of Surgical Patches Benefits
Low Risk of Inflammatory Response
Through a proprietary manufacturing process, the intrinsic nature of the bovine pericardum material is maintained while meeting high levels of biocompatibility and demonstrating a low risk of inflammatory response.2,3,17-20
Strength, Durability and Excellent Handling
The Tissue Guard family of surgical patches are similar to autologous tissue and are easy to handle and suture through; they resist suture line bleeding with excellent reapproximation2-4,10
Multiple Size Options
The Tissue Guard family of surgical patches offer multiple size options and readily conform to multiplanar shapes.
Indications and Important Risk Information
DURA-GUARD
INDICATIONS FOR USE
DURA-GUARD Dural Repair Patch is intended for use as a dura substitute for the closure of dura mater during neurosurgery.
SELECT IMPORTANT RISK INFORMATION DURA-GUARD is not designed, sold or intended for use except as indicated.
SELECT WARNINGS Failure to rinse the product may result in a sterile inflammatory reaction. Do Not freeze. The patch must remain moist at all times.
Rx only. For safe and proper use of this device refer to the complete Instructions for Use.
VASCU-GUARD
Indications For Use
Vascu-Guard Vascular Patch is used for peripheral vascular reconstruction including the carotid, renal, iliac, femoral, profunda and tibial vessels and arteriovenous revisions.
SELECT IMPORTANT RISK INFORMATION
VASCU-GUARD is not designed, sold or intended for use except as indicated.
SELECT WARNINGS Failure to rinse the product may result in a sterile inflammatory reaction. Do Not freeze. The patch must remain moist at all times.
Rx only. For safe and proper use of this device refer to the complete Instructions for Use.
PERI-GUARD
INDICATIONS FOR USE
Peri-Guard Repair Patch (PERI-GUARD) is intended for use as a prosthesis for surgical repair of pericardial structures and soft tissue deficiencies which include: defects of the abdominal and thoracic wall, gastric binding, muscle flap reinforcement, and hernias (diaphragmatic, femoral, incisional, inguinal, lumbar, scrotal, and umbilical hernias). Peri-Guard is also intended for use as a patch material for intracardiac defects, great vessel, septal defects and annulus repair, and suture-line buttressing.
SELECT IMPORTANT RISK INFORMATION
PERI-GUARD is not designed, sold or intended for use except as indicated.
SELECT WARNINGS Failure to rinse the product may result in a sterile inflammatory reaction. Do Not freeze. The patch must remain moist at all times.
Rx only. For safe and proper use of this device refer to the complete Instructions for Use.
SUPPLE PERI-GUARD
INDICATIONS FOR USE
SUPPLE PERI-GUARD is intended for use as a prosthesis for pericardial closure and soft tissue deficiencies which include: defects of the abdominal and thoracic wall, gastric banding, muscle flap reinforcement, and hernias (diaphragmatic, femoral, incisional, inguinal, lumbar, scrotal and umbilical).
SELECT IMPORTANT RISK INFORMATION
SUPPLE PERI-GUARD is not designed, sold or intended for use except as indicated.
SELECT WARNINGS Failure to rinse the product may result in a sterile inflammatory reaction. Do not freeze. The patch must remain moist at all times.
Rx only. For safe and proper use of this device refer to the complete Instructions for Use.
TISSUE GUARD Family of Surgical Patches Full Instructions for Use: