Choosing a Fibrin Sealant: TISSEEL [Fibrin Sealant] vs Vistaseal Fibrin Sealant
O.R. Insights Blog
CHOOSING A FIBRIN SEALANT: TISSEEL [FIBRIN SEALANT] VS. VISTASEAL FIBRIN SEALANT
Hemostasis and sealing represent crucial steps toward successful surgical outcomes.1,2 There are many hemostatic products available today, including fibrin sealants, which are utilized for hemostasis and tissue sealing across a range of surgical procedures, including cardiothoracic, general (e.g., bariatric, colorectal, hepatic), gynecologic, orthopedic, oncologic, plastic, trauma, urologic, and vascular surgery. 1,2 Two currently available fibrin sealants are Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant.3,4
This blog will explore the composition, efficacy, versatility and safety profiles of these fibrin sealants, and help you understand key differences between these two products. Here, Amit K. Taggar, MD*, a general surgeon specializing in bariatric surgery at Advent Health in Celebration, Florida, provides an overview of both products to guide you in making an informed decision tailored to your surgical needs.
Introduction
Fibrin sealant is a two-component topical hemostat, sealant and tissue adhesive consisting of fibrinogen and thrombin that has been used in the United States (US) as a blood bank- or laboratory-derived product since the 1980s.5 Fibrin sealants have been commercially available in the US since 1998.5 These agents exploit the final stage of the coagulation cascade.3 In the presence of small amounts of calcium and Factor XIII, thrombin converts fibrinogen into insoluble fibrin, the final stable form of the agent.6
The use of fibrin sealants has become common surgical practice across a wide range of surgical disciplines including cardiothoracic, general (e.g., bariatric, colorectal, hepatic), gynecologic, orthopedic, oncologic, plastic trauma, urologic, and vascular surgery.1,2 The versatility of fibrin sealants, including those with both pooled human plasma and bovine plasma derivatives, underscores their importance in modern surgery and potential for future medical innovations.5
- It must be effective
- The product should be safe with no significant adverse effects associated with application or it’s degradation products
- Should be able to enhance clot formation and wound healing
- Should polymerize quickly and be reasonably priced
Two leading fibrin sealants, Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant, have gained significant recognition in the field, each with its unique set of features and benefits.3,4 Choosing between these two prominent options can pose a challenge for medical professionals. This blog aims to dissect this decision-making process, providing an in-depth comparison of Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant.
What Are Fibrin Sealants?
A fibrin sealant was first used for hemostasis in 1909.6 However, it was not until 1940 that the tissue adhesive properties of fibrinogen were described.2 The development of fibrin sealant since the 1970s has been gradually advanced and optimized; the use of concentrated plasma fibrinogen and bovine/human thrombin has led to the widespread use and approval of fibrin sealant in surgical procedures.3,4,7
The development and application of fibrin sealants have been significantly influenced by regulatory approval and clinical research. The evolution from non-commercial, autologous fibrin sealants to commercial products highlights the ongoing enhancement of fibrin sealant formulations, addressing both safety and efficacy concerns.2,5 The US Food and Drug Administration (FDA) has approved these products for multiple specific uses, such as achieving hemostasis during surgery and sealing colonic anastomoses, rhytidectomy and attaching skin grafts in burn patients.3,6 Fibrin sealant is the only commercially‑available FDA‑approved material for clinical use as a hemostat, sealant and adhesive (Figure 1).6
Figure 1: Fibrin Sealants: Approved Indications6
A Physiological Choice for Hemostasis and Sealing
Fibrin sealants play a critical role in controlling blood and fluid loss, which is particularly beneficial in surgeries involving tissues that are challenging to suture.2-4,5 Fibrin sealants are recognized for their active, physiological approach to hemostasis and sealing due to their mimicry of the final stages of the natural coagulation cascade.3 This process results in the formation of a stable fibrin clot, closely aligning with the body's inherent mechanisms for bleeding control and wound healing. 2 Alternatively, passive agents (e.g. oxidized cellulose, collagen, gelatin, etc.), or mechanical hemostats, provide a physical structure for platelet activation and clot formation.1 Further they rely on the patient’s ability to generate clotting factors.1 Thus, passive products are most effective for minimal bleeding scenarios and are appropriate for patients who have an adequately functioning coagulation cascade.1 In addition, by offering an active, physiological approach to hemostasis and tissue sealing, fibrin sealants also present a biocompatible alternative to synthetic adhesives, avoiding the issues of inflammation and tissue necrosis.2,8
Fibrin sealants can be used for hemostasis, wound closure and tissue sealing and have been advocated as the agents that are closest to approaching the ideal operative sealant (see Figure 1).8 In contrast to synthetic adhesives, fibrin sealants have the advantage of being biocompatible and biodegradable, and they are not associated with inflammation, foreign body reactions, tissue necrosis or extensive fibrosis.8 Reabsorption of the fibrin clot is achieved during normal wound healing within days to weeks of application.2,3
The biocompatibility of fibrin sealants, derived from human plasma components, ensures minimal inflammatory response.8 This aligns with the medical aim to reduce surgical blood and fluid loss, making fibrin sealants a preferred choice for a wide range of surgical applications.1
Manufacturing and Pathogen Safety
Fibrin sealants are derived from pooled and individual human plasma.9 Manufacturing of fibrin sealants involves a meticulous process that starts with the purification of its main components (fibrinogen, thrombin, and sometimes Factor XIII) from human plasma, and in some cases, includes bovine aprotinin.10 The manufacturing steps are designed to minimize the risk of viral transmission, incorporating various procedures like heat treatment (e.g., pasteurization, dry or vapor heating), filtration, solvent/detergent treatment, precipitation, pH adjustment, and chromatography. These steps not only purify the components but also specifically target the inactivation or elimination of potential pathogens, ensuring the production of a safe product.10
The safety of fibrin sealants against pathogens is further enhanced by rigorous selection and screening of plasma donors using sensitive molecular techniques, such as polymerase chain reaction (PCR) testing, to reduce the theoretical possibility of viral transmission.10 The manufacturing process is validated through a series of tests that assess the effectiveness of each step in removing or inactivating viruses.10
Introduction to Tisseel [fibrin sealant]
Tisseel [fibrin sealant] was the first fibrin sealant approved in the US in 1998 following a prospective, randomized, multicenter clinical trial in cardiac surgery conducted by Rousou et al.3,11 Dr. Rousou credits the work of Dr. Borst and colleagues in Germany in the early 1980s for inspiring him to seek approval of the product in the US. 3,11
Tisseel [fibrin sealant] is indicated for use as an adjunct to hemostasis in adult and pediatric patients (>1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical.3 Tisseel [fibrin sealant] is effective in heparinized patients and is also indicated as an adjunct to standard surgical techniques (such as suture and ligature) to prevent leakage from colonic anastomoses following the reversal of temporary colostomies.3
Composition of Tisseel [fibrin sealant]
Tisseel [fibrin sealant] is a two-component fibrin sealant made from pooled human plasma.3 When combined, the two components, a human sealer protein and human thrombin mimic the final stage of the blood coagulation cascade.3 The sealer protein is a sterile, non-pyrogenic, vapor heated and solvent/detergent treated preparation made from pooled human plasma. The sealer protein also contains a fibrinolysis inhibitor, synthetic aprotinin, that is synthetic and completely of non-human/non-animal origin.3 The thrombin is also a sterile, non-pyrogenic, vapor heated and solvent/detergent treated preparation made from pooled human plasma. In the presence of small amounts of calcium and Factor XIII, the thrombin converts fibrinogen into insoluble fibrin, the final stable form of the agent.6 Details of the composition of Tisseel [fibrin sealant] are provided in Figure 2.3
Figure 2: Tisseel [fibrin sealant] Composition: Dual-Chambered Syringe3
How Tisseel [fibrin sealant] Works
Upon mixing the human sealer protein and thrombin, soluble fibrinogen is transformed into fibrin, forming a rubber-like mass that adheres to the wound surface and achieves hemostasis and sealing or gluing of tissues.3 Tisseel [fibrin sealant] mimics the final coagulation cascade step as it has all relevant components to form a clot (Video 1).3 Thrombin is a highly specific protease that transforms the fibrinogen contained in Sealer Protein into fibrin. Fibrinolysis inhibitor, aprotinin (synthetic), is a polyvalent protease inhibitor that prevents premature degradation of fibrin. Preclinical studies have shown that incorporation of aprotinin increases resistance of the fibrin sealant clot to degradation in a fibrinolytic environment.3
Clinical Evidence for Use of Tisseel [fibrin sealant] in Surgical Practice
The use of Tisseel [fibrin sealant] has been extensively studied in a wide variety of surgical specialties including cardiothoracic, general (e.g., bariatric, colorectal, hepatic), gynecologic, orthopedic, oncologic, plastic, trauma, urologic, and vascular surgery.1-3 In addition, published clinical research for fibrin sealants spans a broad range of surgical disciplines, emphasizing its multifaceted utility.6 This diversity of fibrin sealant applications, and the approval of Tisseel [fibrin sealant], highlight its capacity to meet the specific requirements of various surgical procedures.3,6
Introduction to Vistaseal Fibrin Sealant
Vistaseal Fibrin Sealant was approved for use in the US in 2017 following a prospective, randomized, multicenter clinical trial in vascular surgery conducted by Chetter et al.4,12
Vistaseal Fibrin Sealant is indicated as an adjunct to hemostasis for mild to moderate bleeding in adults undergoing surgery when control of bleeding by standard surgical techniques (such as suture, ligature and cautery) is ineffective or impractical.4 Vistaseal Fibrin Sealant is effective in heparinized patients.4
Composition of Vistaseal Fibrin Sealant
Vistaseal Fibrin Sealant is a two-component fibrin sealant consistent of human fibrinogen (component 1 [sterile solution]) and human thrombin with calcium chloride (component 2 [sterile solution]).4
Chamber Components:4
- Chamber 1: Thrombin Human: 500 units/mL
- Chamber 2: Fibrinogen Human: 80mg/mL
How Vistaseal Fibrin Sealant Works
Vistaseal Fibrin Sealant contains human fibrinogen and human thrombin.4 Similar to Tisseel [fibrin sealant] (see Figure 2),3 when applied onto the wound site and mixed, these biological components generate a cross-linked fibrin clot in a process that recreates the last stage of the human blood coagulation system.4 Fibrinogen is converted into fibrin monomers and fibrinopeptides by thrombin.4 The fibrin monomers aggregate and form a fibrin clot which stops the bleeding.4 Factor XIIIa, which is activated from Factor XIII by thrombin, crosslinks fibrin.4 Calcium ions are required for both the conversion of fibrinogen and the crosslinking of fibrin.4
Clinical Evidence for Use of Vistaseal Fibrin Sealant in Surgical Practice
Vistaseal Fibrin Sealant was approved in 2017.4 As mentioned above, Vistaseal Fibrin Sealant was evaluated in a prospective, randomized, multicenter clinical trial in vascular surgery conducted by Chetter et al.12 Following publication of these pivotal study results, the product received FDA approval.4
Comparison of Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant
While Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant are similar, there are key differences.3,4 In this section, we’ll explore these differences and the potential impacts on clinical use.
Importantly, Tisseel [fibrin sealant] is indicated for hemostasis, sealing and in pediatric patients (>1 month of age), while Vistaseal Fibrin Sealant is only indicated for hemostasis and does not currently have an indication for sealing nor in pediatric patients.3,4
Clot Formation Comparison
When comparing scanning electron microscope (SEM)(x10,000) images of the clots formed by Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant, as compared to a physiologic clot, the clot formed by Tisseel [fibrin sealant] closely resembled the physiologic clot, while the Vistaseal Fibrin Sealant clot is lacking well-defined fibrin strands and the open pore structure of the physiologic clot (Figure 4).13-15
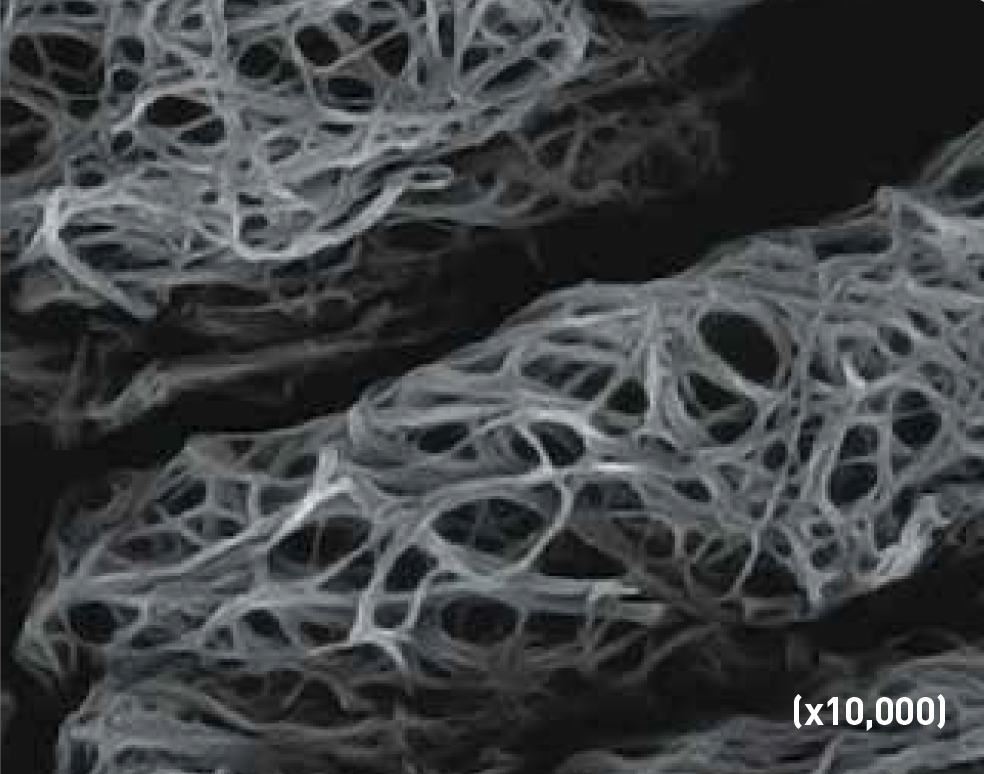
Normal Physiologic Clot
An open-pore structure enables diffusion of nutrients, stem cells, an in-growth of fibroblasts which helps promote the body's natural wound-healing process.

TISSEEL (fibrin sealant) Clot
Tisseel (fibrin sealant) shows a clot structure that closely resembles a normal physiologic clot.

Vistaseal Fibrin Sealant Clot
Compared to Tisseel (fibrin sealant) Vistaseal fibrin sealant is least similar to a normal physiologic clot, lacking well-defined fibrin strands and open-pore structure.

Product Composition Comparison
As detailed in Figure 2, Tisseel [fibrin sealant] contains synthetic aprotinin which is a clot stabilizer that prevents premature clot degradation.3 Vistaseal Fibrin Sealant does not contain a clot stabilizer.4 Tisseel [fibrin sealant] is expected to remain in the body for 10 to 14 days.3 The clot stability and longer time to clot degradation as a result of synthetic aprotinin in Tisseel [fibrin sealant] allows for sufficient time during the critical wound healing period.3

Spray Coverage Comparison
As detailed in the prescribing information for both products, Tisseel [fibrin sealant] has broader spray coverage as compared to Vistaseal Fibrin Sealant (Figure 5).3,4 A 4 mL syringe of Tisseel [fibrin sealant] covers an area of approximately 200 cm2 while a similar size of Vistaseal Fibrin Sealant only covers approximately 32-44 cm2.3,4
Figure 5: Comparison of Spray Coverage for Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant3,4
Summary
Although Tisseel [fibrin sealant] and Vistaseal Fibrin Sealant share similarities, they exhibit key product differences that impact their clinical application and performance.3,4 Tisseel [fibrin sealant], approved in 1998, offers a broader indication for use in both hemostasis and sealing (to prevent leakage from colonic anastomoses following the reversal of temporary colostomies) across a wide patient demographic, including adults and pediatric patients (>1 month of age), whereas Vistaseal Fibrin Sealant, approved in 2017, is limited to hemostasis in adult patients only.3,4 This difference highlights the extensive clinical experience and versatility amassed by Tisseel [fibrin sealant] over the years. 3,4
Moreover, Tisseel [fibrin sealant] demonstrates a unique ability to form a clot that closely mimics the natural physiological process, a feature further enhanced by the inclusion of synthetic aprotinin—a clot stabilizer that ensures clot integrity during the critical phases of wound healing.3,4 This contrasts with Vistaseal Fibrin Sealant, which lacks a clot stabilizer.3,4 Additionally, the greater spray coverage of Tisseel [fibrin sealant], which has approximately four times the spray coverage area compared to Vistaseal Fibrin Sealant, reduces the need to open multiple products, making it a more efficient option.3,4
Given these distinctions, the choice of fibrin sealant should be guided by a thorough understanding of the unique properties and specific clinical scenarios. The broader indication, clot stability and greater spray coverage of Tisseel [fibrin sealant] make it a versatile and reliable option in surgical hemostasis and tissue sealing.3,4 This comparative analysis underscores the importance of meticulous product selection in achieving optimal surgical outcomes, reinforcing the value of innovation and experience in the development of fibrin sealants.
*Dr. Taggar was compensated for his time developing this blog.
TISSEEL (fibrin sealant) Indications
Hemostasis: TISSEEL is a fibrin sealant indicated for use as an adjunct to hemostasis in adult and pediatric patients (> 1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. TISSEEL is effective in heparinized patients.
Sealing: TISSEEL is a fibrin sealant indicated as an adjunct to standard surgical techniques (such as suture and ligature) to prevent leakage from colonic anastomoses following the reversal of temporary colostomies.
Important Risk Information for TISSEEL [Fibrin Sealant]
For Topical Use Only. Do not inject TISSEEL directly into the circulatory system or into highly vascularized tissue. Intravascular application of TISSEEL can lead to intravascular coagulation, can result in life- threatening thromboembolic events, and can increase the likelihood and severity of acute hypersensitivity reactions in susceptible patients. To minimize the risk of intravascular application, exercise caution when using TISSEEL in surgery.
Do not use TISSEEL in individuals with a known hypersensitivity to aprotinin.
Do not use TISSEEL for treatment of severe or brisk arterial or venous bleeding. In these situations, TISSEEL will be washed away in the flow of blood before hemostasis can be attained.
Do not spray TISSEEL where the minimum recommended distance from the applicator tip to the target site cannot be assured.
Hypersensitivity or allergic/anaphylactoid reactions can occur with the use of TISSEEL. Such reactions may especially be seen if TISSEEL is applied repeatedly over time or in the same setting, or if systemic aprotinin has been administered previously.
Aprotonin is known to be associated with anaphylactic reactions. Even in the case of strict local application of aprotinin, there is a risk of anaphylactic reactions to aprotinin, particularly in the case of previous exposure.
Discontinue administration of TISSEEL in the event of hypersensitivity reactions. Remove remaining product from the application site.
Air or gas embolism has occurred when fibrin sealant was administered using pressurized gas. This can occur if a spray device is used at higher than recommended pressures and in closer than recommended proximity to the tissue surface.
When using the EASYSPRAY device, or an equivalent spray device for open surgical procedures cleared by FDA, TISSEEL must not be sprayed in enclosed body areas and must be sprayed onto only visible application sites.
TISSEEL is denatured when exposing to solutions containing alcohol, iodine or heavy metals. If any of these substances have been used to clean the wound area, the area must be thoroughly rinsed before the application of TISSEEL.
Apply TISSEEL as a thin layer by dripping or spraying using cannula or spray set. Excess clot thickness can negatively interfere with wound healing.
The safety and effectiveness of TISSEEL used alone or in combination with biocompatible carriers in neurosurgical procedures or other surgeries involving confined spaces have not been evaluated; its use in this setting is not FDA approved.
TISSEEL is made from human plasma. It may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Please see accompanying full Prescribing Information
Baxter and Tisseel are registered trademarks of Baxter International Inc., or its subsidiaries.
Vistaseal is a registered trademark of Ethicon Inc.