The Role of Hemostasis in Cardiovascular Surgery
O.R. Insights Blog
The Role of Hemostasis in Cardiovascular Surgery
Bleeding is a common complication in cardiovascular surgery, worsens the patient’s prognosis, and is often caused by perioperatively acquired hemostatic abnormalities.1 Significant blood loss leads to transfusion, but transfusion is also independently associated with negative outcomes.2 Thus, bleeding is detrimental and costly, and prevention of bleeding can be expected to have tremendous effects on reallocation of resources.2
This blog will explore the topic of hemostasis in cardiovascular surgery, delving into the complexities of bleeding control during these critical procedures. Here, Torben Colberg, MD, MBA, Vice President of Advanced Surgery Worldwide Medical at Baxter Healthcare, provides an overview of the intricacies of hemostasis, highlighting the significance of key technologies and approaches in enhancing surgical safety and effectiveness.
Introduction
Effective hemostasis as part of a blood management strategy is a critical requirement for patients undergoing surgery.3 A variety of principles help to guide surgeons and operating room (O.R.) staff in achieving hemostasis during cardiovascular procedures. Depending on the procedure and circumstances, a range of approaches may be used. Keep reading to learn more about the role of hemostasis in cardiovascular surgery. We will begin with foundational information, then focus on specifics techniques and interventions, including topical hemostatic agents.
Understanding the Foundations: Cardiovascular Surgery and Hemostasis
Cardiovascular Surgery: A Primer
Cardiovascular surgery encompasses a broad range of procedures aimed at treating diseases and conditions affecting the heart and blood vessels.4 This includes coronary artery bypass grafting (CABG) for coronary artery disease, heart valve repair or replacement, aneurysm repair, heart transplantation, insertion of a ventricular assist device, and surgeries for congenital heart disease, among others.5,6 These procedures are critical for addressing cardiovascular disease, the leading cause of morbidity and mortality worldwide, and rising health care costs.4,7
The Essence of Hemostasis in Surgery
Hemostasis, in the broadest sense, refers to the process of cessation of bleeding.8 A more refined clinical definition of hemostasis is bleeding control without the induction of pathologic thrombotic events such as myocardial infarction, stroke, arterial thrombosis, or deep vein thrombosis (Figure 1).8 The process involves a complex cascade of events leading to the formation of a stable blood clot, including vascular constriction, activation of coagulation factors, and platelet plug formation.9
In the surgical context, achieving hemostasis is paramount to prevent excessive blood loss, which can compromise patient recovery and outcomes.10,11 In the United States, national data has shown that cardiac surgical procedures utilize somewhere between 10 and 15% of the approximately 15 million units of packed red blood cells received by surgical patients annually.12

Figure 1: Normal Hemostasis: A Result of Interaction Among Physiologic Systems that Promote Bleeding (Anticoagulant and Fibrinolytic) or Clotting (Procoagulant and Antifibrinolytic)8
Figure adapted from Levy et al8
Acquired Hemostatic Abnormalities in Cardiovascular Surgery
In the realm of cardiovascular surgery, factors contributing to acquired hemostatic abnormalities include the use of anticoagulants as well as the activation and consumption of coagulation factors and platelets induced by cardiopulmonary bypass (CPB).1
To counteract the stimulation of intrinsic and extrinsic coagulation pathways as a result of CPB, patients are systematically heparinized.12 The pathophysiology of bleeding after CPB is multifactorial and complex, involving hypothermia, hemodilution, activation of coagulation, endothelial cell and tissue injury, foreign-surface contact, consumption of clotting factors, platelets activation, platelets dysfunction and hyperfibrinolysis.13
Critical Nature of Limiting Bleeding
Bleeding can be a complication of surgery that may lead to substantial morbidity and mortality.14 In cardiac surgery, severe bleeding occurs in approximately 7% of cases and is associated with an increased risk of postoperative mortality.14 Limiting bleeding during cardiac surgery is vital to maintaining the patient's hemodynamic stability and minimizing the risk of transfusion-related complications.13

Blood transfusions occur in approximately 21% of all operations, and in 45.8% of cardiac cases; however, they are associated with multiple risks and complications.15
Acutely, bleeding can lead also to1
-
hemorrhagic shock
-
cardiac tamponade, or
-
cardiac decompensation
In addition, patients undergoing cardiac surgery are prone to perioperative hemostatic abnormalities requiring surgical revision in 2% to 6% of patients.1 Re-exploration for bleeding worsens the patient’s prognosis with a 3- to 4-fold increase of mortality.1 There are also significant increases in costs in patients with bleeding-related complications.14
Complications from Inadequate Hemostasis
Inadequate surgical hemostasis may lead to transfusion and/or other bleeding-related complications.14 Failure to achieve hemostasis in cardiac surgery can, for example, lead to retained blood which, while difficult to quantify, can result in a variety of negative postoperative outcomes.16
Complications of Retained Blood in the Chest Following Cardiac Surgery:
- Development of cardiac tamponade16
- Low cardiac output16
- Chronic pleural effusions16
- Extended or prolonged monitoring in the intensive care unit16
- Prolonged drainage with chest tubes which can result in increased postoperative pain and the need for narcotics16
- Potential delay in post-operative extubation while monitoring bleeding which can result in a variety of issues and complications from prolonged mechanical ventilation16
- Mediastinitis16
- Acute pericarditis with associated/confounding electrocardiogram changes and/or arrhythmias16
- Long-term development of constrictive pericarditis16, 17
Unless coagulopathy is present, bleeding starts with vessel interruption. If surgical repair occurs in a reasonable period of time, the bleeding is contained, and hemostasis is secured, no additional action is needed.2 If repair is delayed, clotting may still occur, but more often, the clots formed are dislodged and bleeding continues or recurs.2
With sustained bleeding, coagulation factors are consumed and hemodilution, hypothermia, and acidosis compound factor consumption and lead to more bleeding.2 The consequences for cellular perfusion and oxygenation of untreated hemorrhage and hypovolemia are well known and recommendations regarding resuscitation are based on the need to maintain adequate cellular perfusion.2
Visual estimation is the most common method to estimate intraoperative blood loss, but it is not the most accurate.18
Monitoring Blood Loss
Estimating blood loss might be difficult, especially if most of the blood is absorbed by surgical gauze and not collected in the suction bottle or cell saver.18,19
Guidelines aimed at improving the management of perioperative coagulopathic bleeding in cardiac surgery recommend the use of point-of-care (POC) hemostatic testing within the context of integrated transfusion algorithms.20,21 Compared with standard hemostatic tests, POC tests have faster turnaround times and better ability to identify specific coagulation defects, thereby allowing for targeted and more rapid management of coagulopathic bleeding.21
Common Methods of Achieving Hemostasis
Surgeons and operating room staff employ various strategies to achieve hemostasis.22 These include mechanical methods (such as sutures and clips), thermal techniques (like electrocautery), and the application of topical hemostatic agents consisting of mechanical, active, flowable, and fibrin sealants.22 The choice of method depends on the specific surgical context and source of bleeding.
The Role of Hemostasis in Cardiovascular Surgery
Hemostasis and the Prevention of Severe or Massive Bleeding in Cardiovascular Surgery
Coagulopathy associated with cardiac surgery is a multifactorial serious complication that may result in massive bleeding requiring transfusion of red blood cells and procoagulant products to obtain adequate hemostasis.23 Severe or massive hemorrhage in cardiac surgery is an infrequent but clinically significant event. 24 Estimations vary considerably (2-10 %) depending on the definition of massive hemorrhage but are nevertheless associated with high mortality.24
Failed or delayed treatment of a massive bleeding can result in irreversible end-organ damage (e.g., renal failure), cardiovascular events (e.g., stroke, myocardial injury) or death, accompanied by significantly increased costs.24 In cardiac surgery, thromboelastometry and thromboelastography, as well as other POC methods of coagulation monitoring, have proven quite effective in predicting excessive hemorrhage, reducing perioperative bleeding, transfused blood products and associated morbidity.24
Achieving Hemostasis: Techniques and Tools
Surgeons have an array of options to control bleeding, including mechanical and thermal techniques and devices as well as pharmacotherapies and topical agents.25 Application of direct pressure or compression at a bleeding site is often the surgeon’s first choice to assist in the control of bleeding.25 Other mechanical methods, including sutures, staples, and ligating clips, are useful if the source of bleeding is easily identifiable and able to be sealed.25 Compression or other mechanical methods, however, may not be appropriate during all surgical procedures; for example in cardiac surgery with heparinized patients.25

The use of biological agents during surgical procedures, and especially in high-risk operations like cardiac surgery, can result in a significant decrease in transfusion rates, hospital lengths of stay, and readmission rates.16 Aggressive and timely control of surgical bleeding can have a major and significant impact on the overall cost of care, particularly by reducing the risks for developing postoperative complications.16
Topical Hemostatic Agents in Cardiovascular Surgery
Absorbable topical hemostatic agents have since been developed and provide useful adjunctive therapy when conventional methods of hemostasis are ineffective or impractical.25 Topical hemostatic agents can be applied directly to the bleeding site and may prevent continuous unrelenting bleeding throughout the entire procedure and into the postoperative recovery period.25
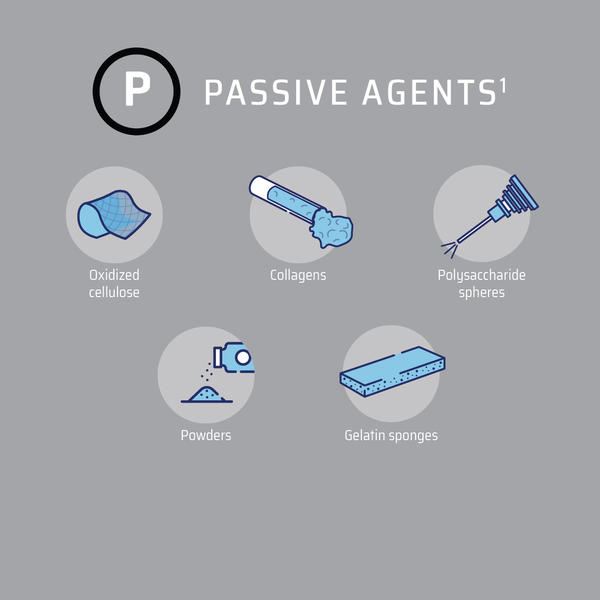
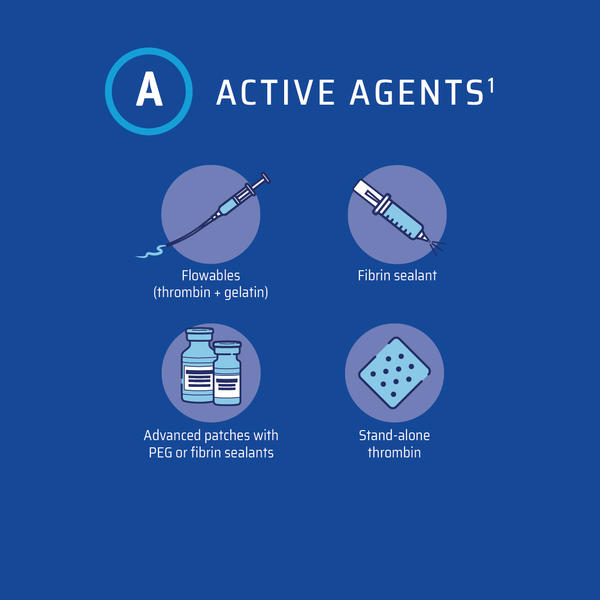
A number of topical hemostatic agents are currently available for use in surgery. They can be divided into two categories: those that provide their mechanism of action on the clotting cascade in a biologically active manner and those that act passively through contact activation and promotion of platelet aggregation (Figure 2).25
Figure 2: Topical Hemostatic Agents3
Figure adapted from Iannitti et al3
Mechanical or passive hemostats form physical barriers that block blood flow and create thrombogenic surfaces, allowing blood to clot more rapidly.3,26 They achieve hemostasis by using patients’ own circulating coagulation factors.3,26 Therefore, they are optimal for patients with an intact coagulation system.3,26
Active products participate at the end of the coagulation cascade and bypass the initial steps of the clotting cascade.3 Thus, other aspects of the coagulation cascade can be dysfunctional without impacting product efficacy.3 Patients with coagulopathies from clotting factors, other than hypofibrinogenemia, platelet dysfunction, or those receiving antithrombotic medications may be ideal candidates for active hemostats.3
Baxter Healthcare understands that during cardiovascular surgery blood management is essential to saving lives and created its portfolio of products to address those needs. Below are a few examples of how Baxter Healthcare products can address bleeding challenges.
Coronary Artery Bypass Graft (CABG)
Floseal Matrix, (Video 1), actively achieves fast hemostasis (median time to hemostasis of 2 minutes*)3,28 supporting improved clinical outcomes28 even in patients who underwent extracorporeal cardiopulmonary bypass,28 and reduced annual cost of care.30
*Median time to hemostasis in minutes for all patients (95% Confidence Interval)
As with other hemostatic agents, do not aspirate Floseal Matrix into extra corporeal cardiopulmonary bypass circuits or autologous blood salvage circuits. It has been demonstrated that fragments of collagen based hemostatic agents may pass through 40 μm transfusion filters of blood scavenging systems.28
In off-pump CABG surgeries, the heart continues to beat while the surgeon performs the bypass. This technique avoids the use of CPB and is beneficial for certain patients.27 However, operating on a beating heart may present unique challenges, particularly in achieving effective hemostasis.
Pericardiectomy
Tisseel [fibrin sealant] can be used to control diffuse bleeding across broad areas in cardiovascular surgery including pericardiectomy and is effective in fully heparinized patients undergoing cardiopulmonary bypass (Video 2). 31
TISSEEL [fibrin sealant] Indications
Tisseel is a fibrin sealant indicated for use as an adjunct to hemostasis in adult and pediatric patients (> 1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. Tisseel [fibrin sealant] is effective in heparinized patients. Click here for Tisseel [fibrin sealant] full Indications, Important Risk Information and full Prescribing Information.
Aortic Aneurysm Repair
During an aortic aneurysm repair, sealants can work independently of the coagulation cascade to prevent bleeding.32-36
Coseal Surgical Sealant, Baxter's fully synthetic sealant, provides a thin, translucent, motion-responsive seal that supports natural vascular dilation and can be sutured through, while allowing for visibility at the suture line (Video 3).34, 35
Preveleak Surgical Sealant contains equal volumes of purified bovine serum albumin and polyaldehyde which rapidly cross link with tissue or graft material to form a strong and stable bond (Video 4).36, 37
Summary and Conclusions
Achieving effective hemostasis in cardiovascular surgery is paramount for ensuring patient safety.1 This article has outlined the foundational knowledge necessary to understand the complexities of hemostasis, detailed the significance of meticulous bleeding control in cardiovascular procedures, and highlighted the innovative hemostatic agents from Baxter Healthcare. As the field of cardiovascular surgery continues to evolve, the focus on refining hemostatic practices remains critical, underscoring the importance of continuous education and adoption of evidence-based strategies to enhance patient care.
FLOSEAL HEMOSTATIC MATRIX INDICATIONS AND IMPORTANT RISK INFORMATION
INDICATIONS
Floseal Matrix is indicated in surgical procedures (other than ophthalmic) as an adjunct to hemostasis when control of bleeding by ligature or conventional procedures is ineffective or impractical.
Important Risk Information
Do not inject intravascularly.
Do not inject or compress Floseal Matrix into blood vessels.
Do not apply Floseal Matrix in the absence of active blood flow, e.g., while the vessel is clamped or bypassed, as extensive intravascular clotting and even death may result.
Do not administer to patients with a history of hypersensitivity to Recothrom Thrombin Topical (Recombinant), any components of Recothrom, or hamster proteins.
Do not use Floseal Matrix in patients with known allergies to materials of bovine origin.
Do not use Floseal Matrix in the closure of skin incisions because it may interfere with the healing of the skin edges due to mechanical interposition of gelatin.
Floseal Matrix is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis. Floseal Matrix is not intended to be used as a prophylactic hemostatic agent.
As with other hemostatic agents, do not apply Floseal Matrix to sites where there is negative peripheral venous pressure, as material may be drawn into the vascular system potentially resulting in life threatening thromboembolic events.
Excess Floseal Matrix (material not incorporated in the hemostatic clot) should always be removed by gentle irrigation and suctioned out of the wound.
The particles of Floseal Matrix swell approximately 10-20% (upon contact with blood or other fluids) and surgeons should consider its potential effect on the surrounding anatomic areas. Maximum swell volume is achieved within about 10 minutes.
Floseal Matrix should not be used in the presence of infection. Floseal Matrix should be used with caution in contaminated areas of the body.
The safety and effectiveness of Floseal Matrix for use in ophthalmic procedures has not been established.
Floseal Matrix should not be used for controlling post-partum bleeding or menorrhagia.
The safety and effectiveness of Floseal Matrix has not been established in children under 2 years of age and pregnant women.
It is not known whether Floseal Matrix can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Floseal Matrix should be administered to a pregnant woman only if clearly needed.
Do not use air to remove residual Floseal Matrix from Applicator tip. The Applicator tips should not be cut, as tissue injury from sharp edges may result.
Hypersensitivity reactions, including anaphylaxis, may occur. Recothrom thrombin is produced in a genetically modified Chinese Hamster Ovary (CHO) cell line and may contain hamster or snake proteins.
For single use only. Do not re-sterilize.
Floseal Matrix should not be applied before the application site is cleaned to remove any antiseptics that may contain alcohol, iodine, or heavy metal ions.
When placed into cavities or closed tissue spaces, gentle approximation is advised. Do not compress.
As with other hemostatic agents, do not aspirate Floseal Matrix into extracorporeal cardiopulmonary bypass circuits or autologous blood salvage circuits.
Do not use Floseal Matrix on bone surfaces where adhesives, such as methylmethacrylate or other acrylic adhesives, will be required to attach a prosthetic device.
Floseal Matrix should not be used for the primary treatment of coagulation disorders.
The safety and effectiveness of the combined use of Floseal Matrix with antibiotic solutions or powders has not been established.
The safety and effectiveness for use in neurosurgical and urological procedures has not been established through randomized clinical studies.
In urological procedures, Floseal Matrix should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
Antibody formation to Recothrom thrombin occurred in <1% of patients. None of the antibodies detected neutralized native human thrombin.
Thrombin must be added to the Gelatin Matrix prior to use.
Rx Only. For safe and proper use of this device, refer to the full Instructions for Use.
TISSEEL (fibrin sealant) INDICATIONS AND IMPORTANT RISK INFORMATION
Indications
Hemostasis: TISSEEL is a fibrin sealant indicated for use as an adjunct to hemostasis in adult and pediatric patients (> 1 month of age) undergoing surgery when control of bleeding by conventional surgical techniques (such as suture, ligature, and cautery) is ineffective or impractical. TISSEEL is effective in heparinized patients.
Sealing: TISSEEL is a fibrin sealant indicated as an adjunct to standard surgical techniques (such as suture and ligature) to prevent leakage from colonic anastomoses following the reversal of temporary colostomies.
Important Risk Information for TISSEEL [Fibrin Sealant]
For Topical Use Only. Do not inject TISSEEL directly into the circulatory system or into highly vascularized tissue. Intravascular application of TISSEEL can lead to intravascular coagulation, can result in life- threatening thromboembolic events, and can increase the likelihood and severity of acute hypersensitivity reactions in susceptible patients. To minimize the risk of intravascular application, exercise caution when using TISSEEL in surgery.
Do not use TISSEEL in individuals with a known hypersensitivity to aprotinin.
Do not use TISSEEL for treatment of severe or brisk arterial or venous bleeding. In these situations, TISSEEL will be washed away in the flow of blood before hemostasis can be attained.
Do not spray TISSEEL where the minimum recommended distance from the applicator tip to the target site cannot be assured.
Hypersensitivity or allergic/anaphylactoid reactions can occur with the use of TISSEEL. Such reactions may especially be seen if TISSEEL is applied repeatedly over time or in the same setting, or if systemic aprotinin has been administered previously.
Aprotonin is known to be associated with anaphylactic reactions. Even in the case of strict local application of aprotinin, there is a risk of anaphylactic reactions to aprotinin, particularly in the case of previous exposure.
Discontinue administration of TISSEEL in the event of hypersensitivity reactions. Remove remaining product from the application site.
Air or gas embolism has occurred when fibrin sealant was administered using pressurized gas. This can occur if a spray device is used at higher than recommended pressures and in closer than recommended proximity to the tissue surface.
When using the EASYSPRAY device, or an equivalent spray device for open surgical procedures cleared by FDA, TISSEEL must not be sprayed in enclosed body areas and must be sprayed onto only visible application sites.
TISSEEL is denatured when exposing to solutions containing alcohol, iodine or heavy metals. If any of these substances have been used to clean the wound area, the area must be thoroughly rinsed before the application of TISSEEL.
Apply TISSEEL as a thin layer by dripping or spraying using cannula or spray set. Excess clot thickness can negatively interfere with wound healing.
The safety and effectiveness of TISSEEL used alone or in combination with biocompatible carriers in neurosurgical procedures or other surgeries involving confined spaces have not been evaluated; its use in this setting is not FDA approved.
TISSEEL is made from human plasma. It may carry a risk of transmitting infectious agents, e.g., viruses, the variant Creutzfeldt-Jakob disease (vCJD) agent and, theoretically, the Creutzfeldt-Jakob disease (CJD) agent.
Please see accompanying full Prescribing Information
COSEAL SURGICAL SEALANT INDICATIONS AND IMPORTANT RISK INFORMATION
INDICATIONS FOR USE
Coseal Surgical Sealant is indicated for use in vascular reconstructions to achieve adjunctive hemostasis by mechanically sealing areas of leakage.
IMPORTANT RISK INFORMATION
Coseal Surgical Sealant is intended for use as an adjunctive sealant and is not to be used in place of sutures, staples or mechanical closure.
Coseal Surgical Sealant swells up to four times its volume within 24 hours of application and additional swelling occurs as the gel resorbs. Therefore, surgeons should consider the maximum swell volume and its possible effect on surrounding anatomic structures potentially sensitive to compression.
Apply only as a thin layer.
Use caution when applying with pressurized gas.
Do not place devices or other objects on top of tissue where Coseal Surgical Sealant has been applied, until the material is fully polymerized (non-tacky). Allow at least 60 seconds after application, and before touching or laying any object on top of the Coseal Surgical Sealant.
Do not apply Coseal Surgical Sealant over any devices or objects that will need to be removed. Coseal Surgical Sealant must not be used as a mechanism of adherence, even temporarily, for any object.
Do not inject Coseal Surgical Sealant into vessels.
The safety and performance of Coseal Surgical Sealant have not been established in children and pregnant women.
In vivo testing demonstrated a mild skin sensitization response in an animal model. Similar testing in humans has not been conducted.
Rx Only. For safe and proper use of this device refer to the complete Instructions for Use.
PREVELEAK INDICATIONS IMPORTANT RISK INFORMATION
INDICATIONS
PREVELEAK Surgical Sealant is indicated for use in vascular and cardiac reconstructions (excluding application to arterial and venous grafts used in coronary artery bypass graft surgery) to achieve adjunctive hemostasis by mechanically sealing areas of potential leakage.
CONTRAINDICATIONS
- Not for use in patients with known allergies to materials of bovine or shellfish origin.
- Not for intravascular use.
- Not for cerebrovascular repair or cerebrospinal leak repair.
IMPORTANT RISK INFORMATION
- Do not use as a substitute for sutures or staples.
- Open lumen procedures require protection of the lumen.
- Avoid exposure to nerves including the sinoatrial node, and the atrial ventricular nodes.
- Do not use in the presence of obvious infection and use with caution in contaminated areas of the body.
- Do not allow either the uncured or polymerized form to come into contact with circulating blood.
- PREVELEAK contains a material of animal origin that may be capable of transmitting infectious agents.
- Repeated use of PREVELEAK in subsequent surgeries has not been studied. Hypersensitivity reactions were not seen using PREVELEAK, but hypersensitivity of BSA has been reported.
- Avoid tissue contact with material expelled from delivery tip during priming.
- Avoid contact with skin or other tissue not intended for application.
- Do not use blood saving devices when suctioning excess PREVELEAK from the surgical field.
- Avoid pausing more than 10-15 seconds between priming and application to prevent polymerization within the delivery tip.
- Minimize use in patients with abnormal calcium metabolism (e.g. chronic renal failure, hyperparathyroidism).
- Safety and effectiveness of PREVELEAK in minimally invasive procedures have not been established.
- Polyaldehyde treated tissue can have an enhanced propensity for mineralization.
- Evidence of cytotoxicity was observed during cell culture-based laboratory assays and is believed to be due to the polyaldehyde component of the product. No evidence of cytotoxicity was observed in animal or clinical studies.
- Do not use PREVELEAK on arterial or venous grafts during coronary artery bypass graft surgery. PREVELEAK may reduce the vasoreactivity of vascular (i.e. internal mammary artery, radial artery or saphenous vein) grafts used in coronary artery bypass graft surgery at the site of application.
USE IN SPECIFIC POPULATIONS
- Use of PREVELEAK in pediatric or pregnant patients has not been studied.
Rx only. For safe and proper use of this device, please refer to the full Instructions for Use.
Baxter, Coseal, Floseal, Preveleak and Tisseel are registered trademarks of Baxter International Inc. or its subsidiaries.