PREVELEAK
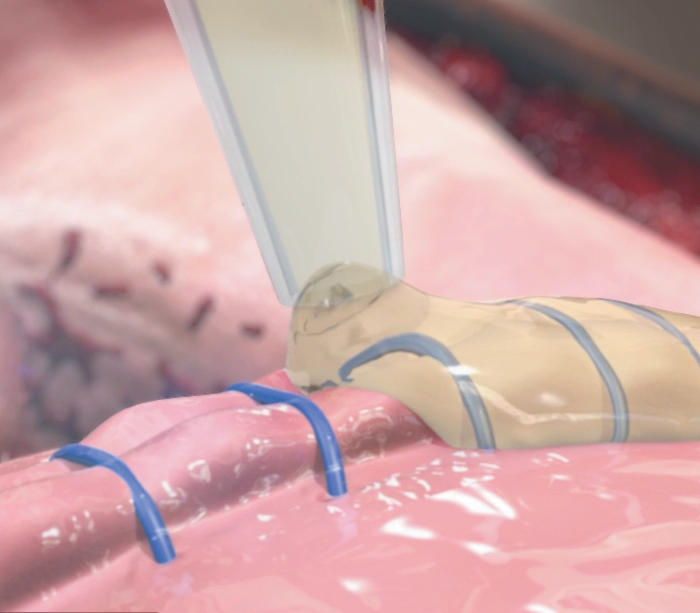
Rapid High-Performance Sealing
PREVELEAK provides rapid sealing with a burst strength in excess of 500 mm Hg*. The strong seal is achieved 60 seconds after application while it gels within 10 to 15 seconds.1,2
*Preclinical data. Results may not correlate to performance in humans
Mechanism of Action
PREVELEAK contains equal volumes of purified bovine serum albumin and polyaldehyde which rapidly cross link with tissue or graft material to form a strong and stable bond. Learn more about how PREVELEAK works.
Additional Product Benefits
Control for Ease-of-Use
High viscosity allows for control during application, minimizing the migration of PREVELEAK surgical sealant from the site of application.
Remains Flexible After Application
PREVELEAK accommodates the natural movement of vessels with a modulus of elasticity similar to that of a healthy human aorta.1
Minimizes Inflammation and Swelling
Proprietary polyaldehyde cross-linker minimizes the inflammatory response associated with glutaraldehyde-based sealants, and the minimal swelling (<10%) of PREVELEAK reduces the potential impact on surrounding structures that may be sensitive to compression.3,4
IMPORTANT SAFETY INFORMATION
PREVELEAK Surgical Sealant Indications
PREVELEAK Surgical Sealant is indicated for use in vascular and cardiac reconstructions (excluding application to arterial and venous grafts used in coronary artery bypass surgery) to achieve adjunctive hemostasis by mechanically sealing areas of leakage.
IMPORTANT RISK INFORMATION
Contraindications
Not for use in patients with known allergies to materials of bovine or shellfish origin.
• Not for intravascular use.
• Not for cerebrovascular repair or cerebrospinal leak repair.
Warnings and Precautions
• Do not use as a substitute for sutures or staples.
• Avoid exposure to nerves.
• Do not use in the presence of obvious infection and use with caution in contaminated areas of the body.
• Do not allow either the uncured or polymerized form to contact circulating blood.
• PREVELEAK contains a material of animal origin that may be capable of transmitting infectious agents.
• Repeated use of PREVELEAK in subsequent surgeries has not been studied.
• Hypersensitivity reactions were not seen using PREVELEAK, but hypersensitivity of BSA has been reported.
• Avoid contact with skin or other tissue not intended for application.
• Safety and effectiveness of PREVELEAK in minimally invasive procedures or coronary artery bypass grafting (CABG) have not been established.
• Do not use blood saving devices when suctioning excess PREVELEAK.
• PREVELEAK syringe and delivery tips are for single patient use only. Do not resterilize.
• Do not use if packages have been opened or damaged.
• Take care not to spill contents of syringe. Avoid tissue contact with material expelled from delivery tip during priming.
• Avoid pausing more than 10-15 seconds between priming and application to prevent polymerization within the delivery tip.
• Minimize use in patients with abnormal calcium metabolism (e.g. chronic renal failure, hyperparathyroidism).
Polyaldehyde treated tissue can have an enhanced propensity for mineralization.
• Evidence of cytotoxicity was observed during cell culture-based laboratory assays and is believed to be due to the polyaldehyde component of the product. No evidence of
cytotoxicity was observed in animal or clinical studies.
Adverse Reactions
• Potential adverse effects associated with the use of this class of surgical sealants include application of the sealant to tissue not targeted for the procedure, failure of the sealant to adhere to the tissue, hypersensitivity reaction such as swelling or edema at the application site, possible transmission of infectious agents from materials of animal origin, thrombosis and thromboembolism.
• Serious adverse events that occurred in clinical studies included death, hypotension, thrombosis/ thromboembolism, ischemia, respiratory failure/dysfunction, steal syndrome, and myocardial infarction.
Use in Specific Populations
• Use of PREVELEAK in pediatric or pregnant patients has not been studied.
Rx only. For safe and proper use of this device, please refer to the full Instructions for Use.