PERI-STRIPS

*PSDV Secure Grip is not approved for lung.
See the Simplicity of PSDV SECURE GRIP on a Medtronic Stapler
PERI-STRIPS DRY Staple Line Reinforcement with VERITAS Collagen Matrix comes with SECURE GRIP Technology, arming you with the power of a biologic material you can rely on combined with the convenience of a faster, simpler preparation.2,3
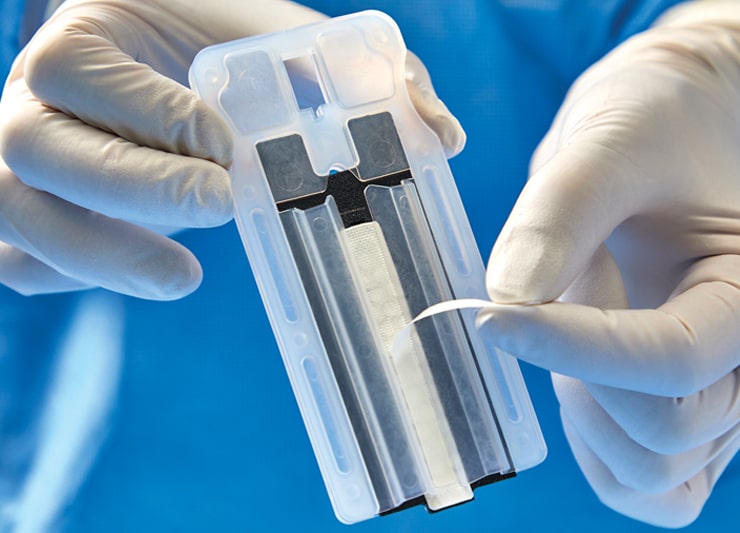
Simple “Peel ‘N Secure” Preparation
The SECURE GRIP technology consists of a pressure-sensitive adhesive that provides optimal adherence and release of the buttress from the stapler, facilitating easy manipulation of tissue.4

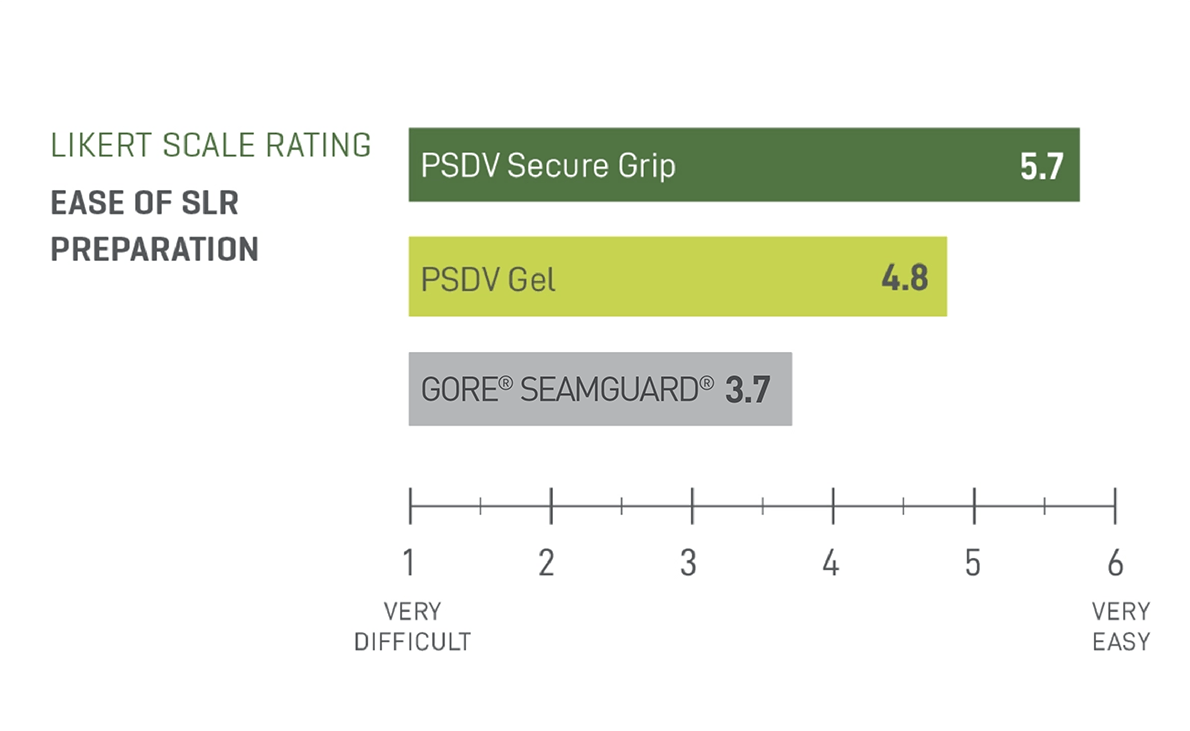
Faster Preparation Time
Half the preparation time of GORE® SEAMGUARD®
Half the preparation of PSDV Gel
PSDV SECURE GRIP resulted in a 54.55% reduction in preparation time when compared to GORE® SEAMGUARD® and a 59.67% reduction in preparation time when compared to PSDV Gel.2
PSDV with Simpler Preparation
Easiest to Prepare, Easiest to Learn2
A straightforward, intuitive preparation that’s easy to adopt.
- Reduces the risk of error2
- Easy for new users to adopt2
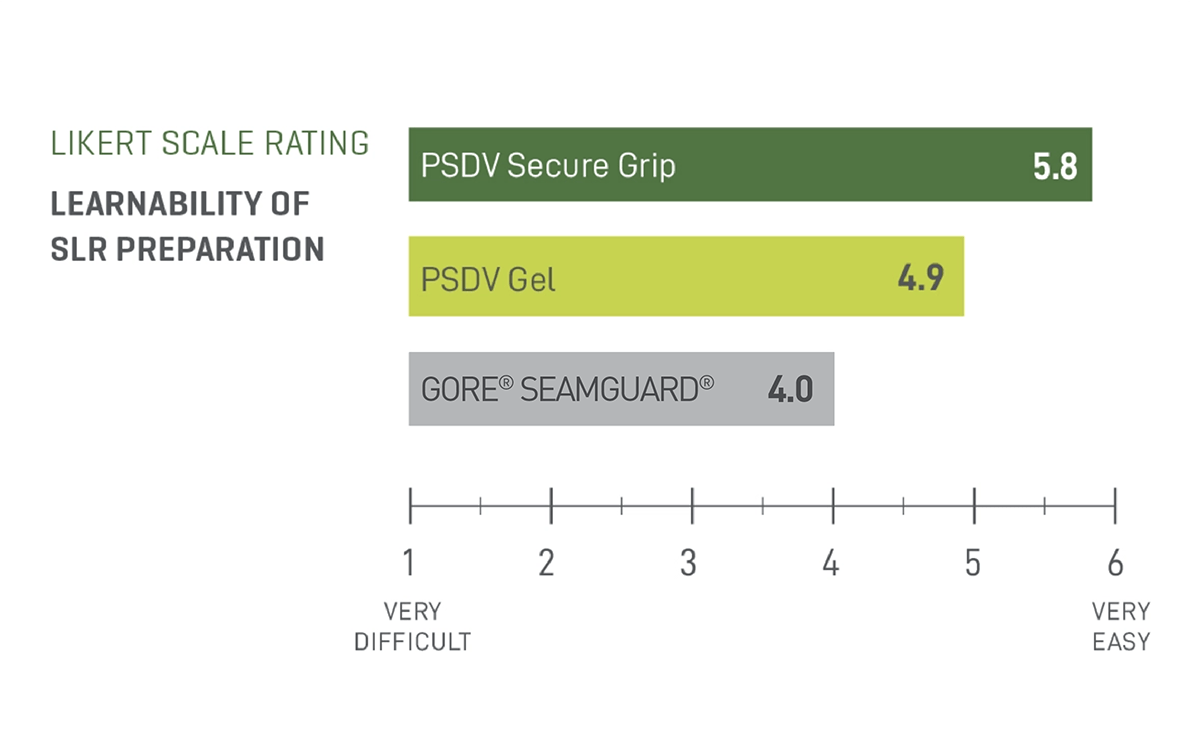
In a preclinical study, participants rated PSDV SECURE GRIP easier to learn than GORE® SEAMGUARD® and PSDV Gel.2
Confidence at the Staple Line
Over 15 years and 2 million implants, PERI-STRIPS DRY with VERITAS is proven to improve clinical outcomes and reduce procedural complications.1,5,6
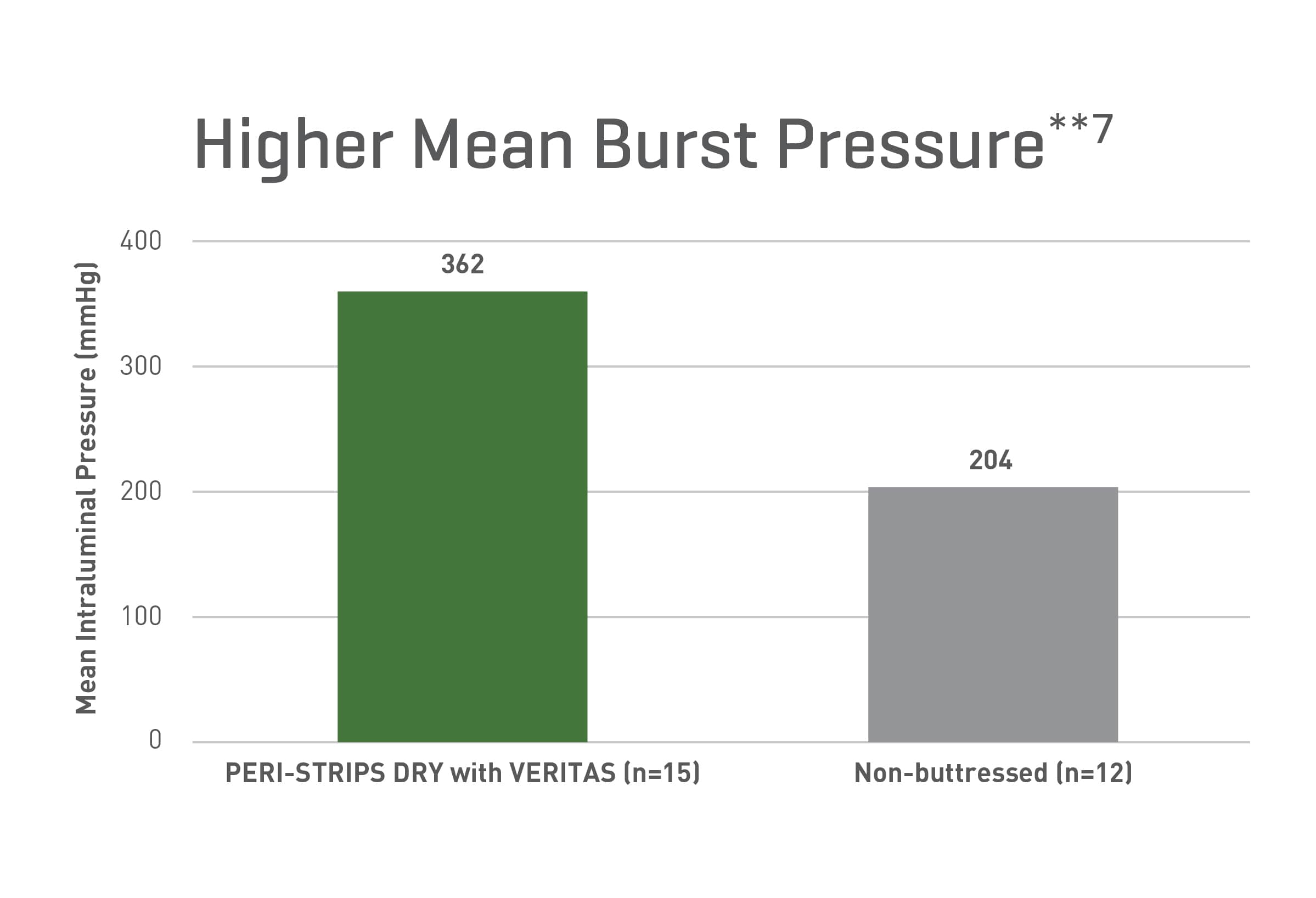
Strong From the Start (Burst Strength)**
A preclinical study evaluating staple line seam integrity at intraluminal pressures in the immediate postoperative period with a canine model demonstrated:
- Regardless of the operative techniques, the buttressed ileocolic anastomoses showed higher bursting pressures**7
- Buttressed anastomoses never ruptured at the staple line**7

The Staple Line Reinforcement MOST PREFERRED by OR Nurses and Scrub Technicians
30 out of 30 nurses and scrub technicians ranked PSDV SECURE GRIP the #1 preferred staple line reinforcement.2
Additional Product Benefits
Outperforms Synthetic PGA Material
PERI-STRIPS DRY with VERITAS is derived from bovine pericardium and outperforms synthetic PGA material at the staple line over time in sleeve gastrectomy/gastric bypass patients.6
Reduced Risk of Staple Line Leaks and Bleeds
In a multicenter comparative use study, PERI-STRIPS DRY with VERITAS significantly lowered the risk of postoperative bleeding and leaks at the staple line compared to competitive products, over-sewing, and no staple line reinforcement.1
Remodeling Characteristics Support Wound Healing
PERI-STRIPS DRY with VERITAS provides structural integrity that supports the host tissue’s formation of new collagen.8 Upon implantation, PSDV acts as a scaffold for extracellular matrix (ECM) deposition and will degrade during the remodeling phase of tissue healing.**9
**Preclinical data results may not correlate to results in humans.
Indications and Important Risk Information
PSDV INSTRUCTIONS FOR USE
PERI-STRIPS DRY with VERITAS Collagen Matrix (PSDV) Reinforcement is intended for use as a prosthesis for the surgical repair of soft tissue deficiencies using surgical staplers when staple line reinforcement is needed.
(Secure Grip only) PSDV-SG is intended for use as a prosthesis for the surgical repair of soft tissue deficiencies using surgical staplers when staple line reinforcement is needed. PSDV-SG can be used for reinforcement of staple lines during bariatric, gastric, small bowel, colon, and colorectal procedures.
(Circular Reinforcement only) PSDV reinforcement can be used for reinforcement of staple lines during gastric, bariatric, and small bowel procedures.
(Linear Reinforcement only) PSDV Reinforcement can be used for reinforcement of staple lines during lung and bronchus resections and during bariatric surgical procedures, and during gastric, small bowel, mesentery, colon, and colorectal procedures.
PSDV SECURE GRIP IMPORTANT RISK INFORMATION
The use of PSDV-SG is contraindicated in patients with known sensitivity to bovine or acrylic material(s).
Do not re-sterilize. Resterilization may cause changes to the tissue and negatively impact functionality of the device.
Discard all open, used, or unused components as sterility is no longer assured.
Do not use product if there is damage to the pouch or seals as sterility may be compromised.
Ensure the staple line is completely covered with the buttress, or it may result in inadequate coverage after firing.
PSDV-SG is not designed, sold, or intended for use except as indicated; doing so may result in surgical complications.
PSDV-SG configurations differ; substitution of one product for another product may reduce product performance.
Do not use PSDV-SG if the product has been submerged in liquids or if heat indicator has been activated.
Use care when removing loading unit components from the stapler to prevent buttress dislodgement.
Do not get the anvil or cartridge buttress wet before applying, or the buttress may not adhere to the stapler properly.
Ensure the anvil and cartridge side of the loading unit are on the corresponding stapler jaws or the buttress may not adhere to the stapler properly.
Final tissue compression, including PSDV-SG, must meet the range specified by the stapler manufacturer; this is especially important if staple firings are overlapped. PSDV-SG increases the total thickness of the area stapled by 0.45 mm–0.86 mm (0.018"–0.033").
Follow Instructions for Use supplied by the stapler manufacturer. Do not use PSDV-SG contrary to the stapler manufacturer’s instructions.
The cartridge and anvil sides of the PSDV-SG loading unit differ; substitution of one side for the other may interfere with alignment and adherence of the buttress strips.
The use of PSDV-SG has not been studied in lung or bronchus resection.
PSDV CIRCULAR IMPORTANT RISK INFORMATION
The use of PSDV-SG is contraindicated in patients with known sensitivity to bovine or acrylic material(s).
Do not re-sterilize. Resterilization may cause changes to the tissue and negatively impact functionality of the device.
Discard all open, used, or unused components as sterility is no longer assured.
Do not use product if there is damage to the pouch or seals as sterility may be compromised.
Ensure the staple line is completely covered with the buttress, or it may result in inadequate coverage after firing.
PSDV-C is not designed, sold, or intended for use except as indicated; doing so may result in surgical complications.
Do not use PSDV-C if heat indicator on the carton label has been activated (i.e. turns red)
Do not get the anvil or cartridge buttress wet before applying, or the buttress may not adhere to the stapler properly.
Cartridge cones may be used for entry into the surgical site on the cartridge side of the stapler during endoscopic surgery. Before completion of surgery be sure to remove the cartridge cone from the surgical site.
Final tissue compression, including PSDV-C, must meet the range specified by the stapler manufacturer. PSDV-C increases the total thickness of the area stapled by 0.4 mm - 1.2 mm (0.016” - 0.048”).
PSDV FULL INSTRUCTIONS FOR USE