RECOTHROM
The ONLY Recombinant Topical Thrombin1
Recombinant human thrombin was developed as an alternative with reduced immunogenicity and infectious risk.3
RECOTHROM is for topical use only – do not inject. You can learn how to reconstitute both configurations of RECOTHROM by downloading the preparation guide, below.

RECOTHROM is for topical use only – do not inject. You can learn how to reconstitute RECOTHROM by watching the preparation videos, below.
Versatility & Convenience

Clinically Proven1
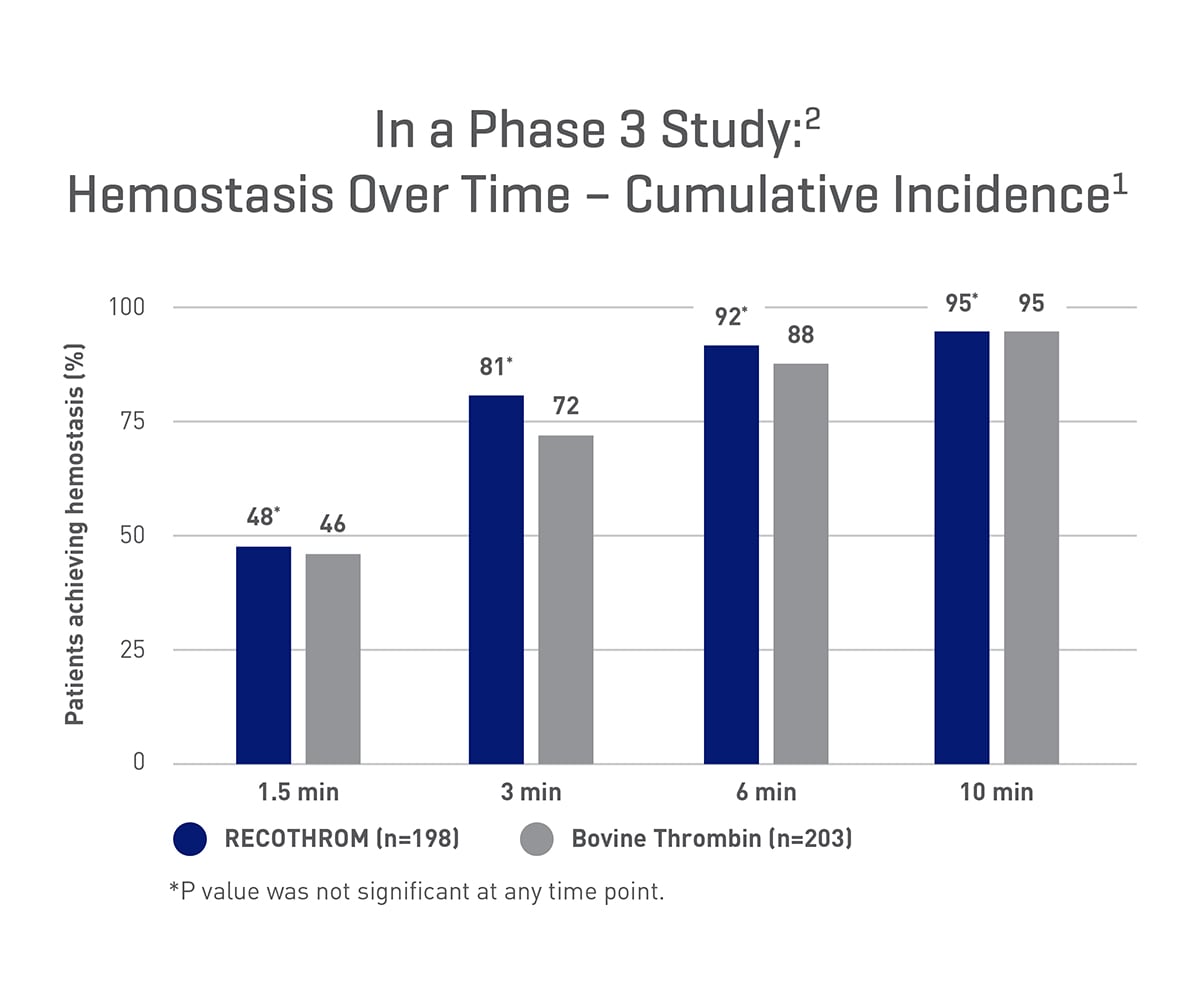
95% of patients treated with RECOTHROM achieved hemostasis within 10 minutes, comparable to bovine thrombin in a non-inferiority study.1,2
In clinical trials, RECOTHROM has been used in patients with or without preexisting bovine or recombinant human thrombin antibodies.1
Additional Product Benefits
Low Antiproduct Antibody Formation
A phase 3 study showed that 1.5% of patients receiving RECOTHROM developed antiproduct antibodies, compared with 21.5% of patients receiving bovine thrombin.1,2*
*This study was not designed or powered to detect an association between antibody formation and adverse clinical outcomes. The overall incidence of AE’s was similar between treatment groups.
Versatile Surgical Applications
Proven efficacy across broad surgical specialties. RECOTHROM may be applied with absorbable gelatin sponge (USP), spray pump, or syringe-tip sprayer.1
A Variety of Configurations
RECOTHROM is available in two sizes: 5,000 units and 20,000 units. An additional spray configuration is available in 20,000 units.1
INDICATIONS AND IMPORTANT RISK INFORMATION
RECOTHROM Thrombin topical (Recombinant) Indication
Recothrom Thrombin topical (Recombinant) is a topical thrombin indicated to aid hemostasis whenever oozing blood and minor bleeding from capillaries and small venules is accessible and control of bleeding by standard surgical techniques (such as suture, ligature, or cautery) is ineffective or impractical in adults and pediatric populations greater than or equal to one month of age.
Recothrom Thrombin topical (Recombinant) may be used in conjunction with an absorbable gelatin sponge, USP.
RECOTHROM Thrombin topical (Recombinant) Important Risk Information
- Do not inject directly into the circulatory system.
- Do not use for the treatment of massive or brisk arterial bleeding.
- Do not administer to patients with a history of hypersensitivity to Recothrom Thrombin topical (Recombinant) or any components of Recothrom or hamster proteins.
- Recothrom Thrombin topical (Recombinant) causes thrombosis if it enters the circulatory system. Apply topically. DO NOT INJECT.
- Hypersensitivity reactions, including anaphylaxis, may occur.
- The most common adverse reaction (incidence 6%) was thromboembolic events.
- Antibody formation to Recothrom Thrombin topical (Recombinant) occurred in <1% of patients. None of the antibodies detected neutralized native human thrombin.
Please see accompanying full Prescribing Information.