Adhesion Prevention: What are my Options in Gynecological Surgery?
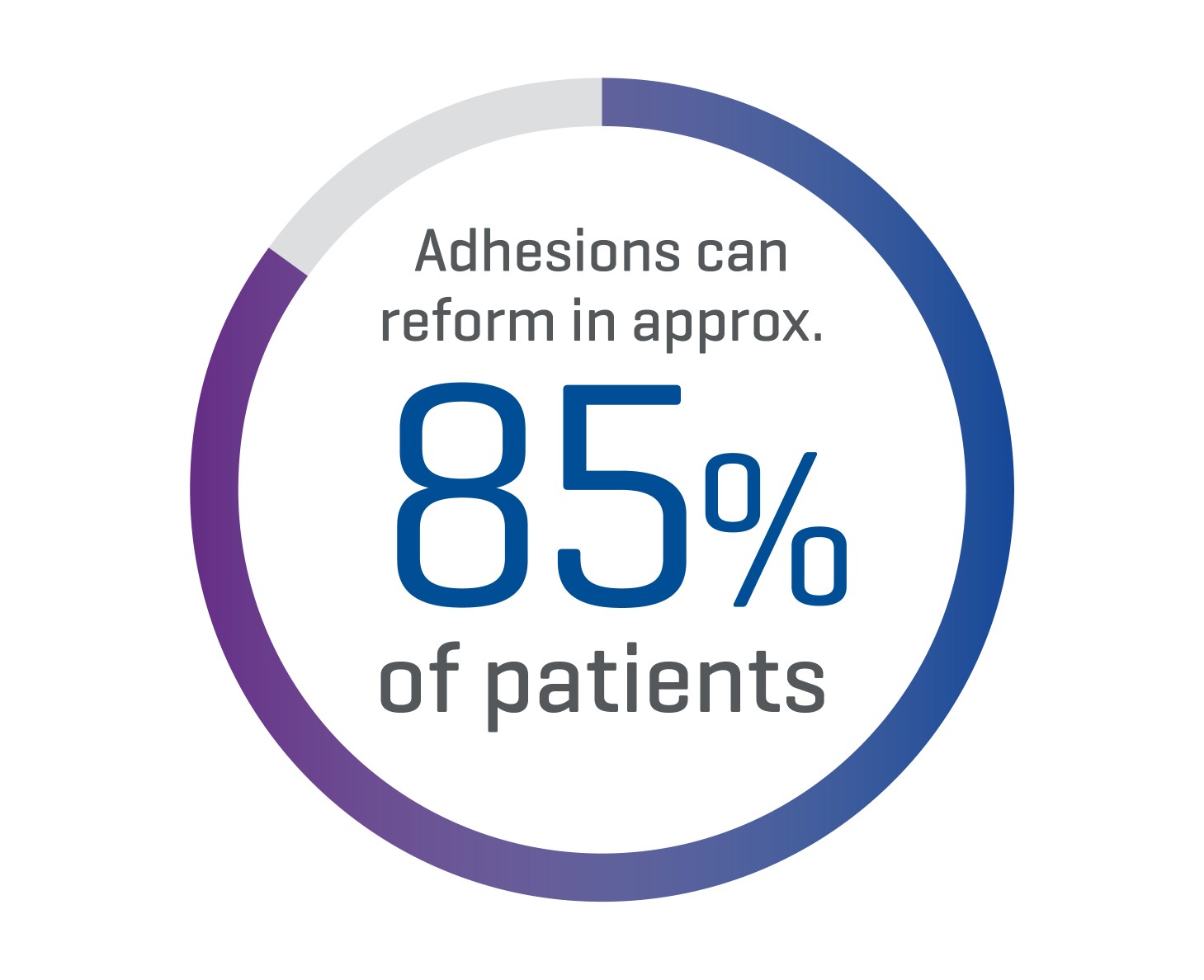
How Common are Adhesions?
Post-surgical adhesion formation is the most common complication of abdominal or pelvic surgery.1 Adhesiolysis is currently the standard treatment approach, but in approximately 85% of patients, adhesions can reform.2,3 Thus, optimal strategies are related to minimizing or preventing adhesion formation in the initial surgery.
Solutions for Adhesion Prevention
Meticulous Surgical Technique4,5
Minimize tissue damage5–7
Gentle tissue handling5–7
Optimal hemostasis5–7
Avoiding tissue desiccation5–7
Preventing thermal injury, foreign body reaction, and infection5–7
A systematic review and meta-analysis of the effect of surgical approach on the incidence of adhesion-related complications found no indication that minimally invasive techniques (e.g., laparoscopy) could improve outcomes5,8
Pharmacological Agents
Suppress inflammatory reactions and pathophysiological processes necessary for the formation of adhesions through the use of drugs targeting adhesion mechanisms4,5,9
Anticoagulants4
Fibrinolytics4
Anti-inflammatory agents4
Antibiotics for the prevention of infection4
Clinical use is limited by potential off target and adverse effects, such as uncontrolled bleeding5
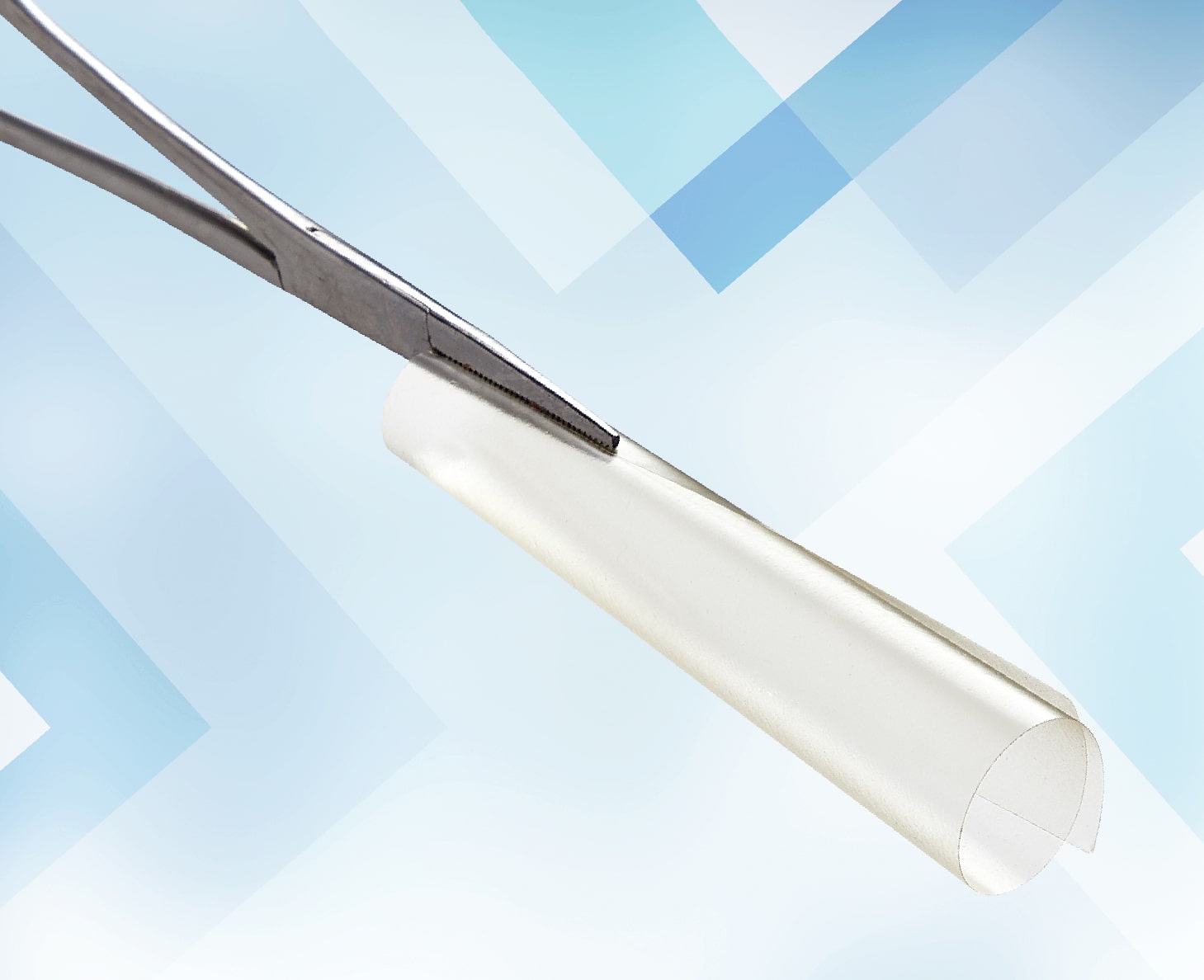
Adhesion Barriers
Natural or synthetic physical separators between surfaces during the healing process can reduce the incidence and severity of post-surgical adhesions4,5,7,10,11
Solid barriers (membranes): SEPRAFILM Adhesion Barrier10
Solutions: ADEPT Adhesion Reduction Solution11
Please see the instructions for use for additional information10
INDICATIONS AND IMPORTANT SAFETY INFORMATION
SEPRAFILM Indications for Use
SEPRAFILM Adhesion Barrier is indicated for use in patients undergoing abdominal or pelvic laparotomy as an adjunct intended to reduce the incidence, extent and severity of postoperative adhesions between the abdominal wall and the under-lying viscera such as omentum, small bowel, bladder, and stomach, and between the uterus and surrounding structures such as tubes and ovaries, large bowel, and bladder.
SEPRAFILM Important Risk Information
SEPRAFILM Adhesion Barrier is contraindicated in patients with a history of hypersensitivity to Seprafilm and/or to any component of SEPRAFILM. SEPRAFILM Adhesion Barrier is contraindicated for use wrapped directly around a fresh anastomotic suture or staple line; as such use increases the risk of anastomotic leak and related events (fistula, abscess, leak, sepsis, peritonitis). SEPRAFILM Adhesion Barrier must be used according to the instructions for use. SEPRAFILM Adhesion Barrier is for single use only, supplied sterile and must not be re-sterilized. Every opened and unused SEPRAFILM pouch must be discarded. Do not use product if pouch is damaged or opened. The number of sheets used should be just adequate to cover the under surface of the abdominal wall or uterine incision in a single layer.
In patients who have ovarian, primary peritoneal or fallopian tube malignancies, SEPRAFILM use has been reported to have an increased risk of intra-abdominal fluid collection and/or abscess, particularly when extensive debulking surgery was required.
The safety and effectiveness of SEPRAFILM Adhesion Barrier has not been evaluated in clinical studies for the following: Patients with frank infections in the abdominopelvic cavity; patients with abdominopelvic malignancy; device placement in locations other than directly beneath an abdominal wall incision following laparotomy, or directly on the uterus following open myomectomy (not laparoscopic); patients with ongoing local and/or systemic inflammatory cell responses; device use in the presence of other implants, e.g. surgical mesh; patients requiring re-operation within four weeks of SEPRAFILM placement – during anticipated time of peak adhesion formation. Foreign body reactions have occurred with SEPRAFILM Adhesion Barrier.
The safety and effectiveness of SEPRAFILM Adhesion Barrier in combination with other adhesion prevention products and/or in other surgical procedures not within the abdominopelvic cavity have not been established in clinical studies.
The safe and effective use of SEPRAFILM Adhesion Barrier in pregnancy and Cesarean section has not been evaluated. No clinical studies have been conducted in pregnant women or women who have become pregnant within the first month after exposure to SEPRAFILM Adhesion Barrier. Therefore, this product is not recommended for use during pregnancy and avoidance of conception should be considered during the first complete menstrual cycle after use of SEPRAFILM Adhesion Barrier.
Long term clinical outcomes such as chronic pain and infertility have not been determined in clinical studies.
Rx Only. For safe and proper use of this device refer to the complete Instructions for Use.
SEPRAFILM Full Instructions For Use
ADEPT® Adhesion Reduction Solution [4% Icodextrin] Indications for Use
ADEPT® Adhesion Reduction Solution is indicated for use intraperitoneally as an adjunct to good surgical technique for the reduction of post-surgical adhesions in patients undergoing gynecological laparoscopic adhesiolysis.
ADEPT® Adhesion Reduction Solution [4% Icodextrin] Important Risk Information
ADEPT® Solution is for direct intraperitoneal administration only. NOT for intravenous (IV) administration.
ADEPT® is contraindicated in patients with known or suspected allergy to cornstarch based polymers e.g. icodextrin, or with maltose or isomaltose intolerance, or with glycogen storage disease.
ADEPT® is contraindicated in laparotomy, in cases involving bowel resection or repair, or appendectomy and in surgical cases with frank abdomino-pelvic infection. There have been rare reports of sterile peritonitis following the use of icodextrin.
Leakage of ADEPT® from port sites may lead to wound healing complications; meticulous fascial closure may reduce leakage through laparoscopic port sites postoperatively. There have been rare reports of hypersensitivity reactions, pulmonary edema, pulmonary effusion and arrhythmia. Anaphylaxis has been reported in a few patients.
Maltose metabolites of icodextrin may interfere with blood glucose measurement in diabetic patients who use rapid blood glucose systems that are not glucose specific.
In the pivotal study, the most frequently occurring treatment related adverse events between surgeries were post procedural leaking from port sites, labial, vulvar or vaginal swelling and abdominal distention.
Rx Only. For safe and proper use of this device, please refer to full Instructions For Use.
