SEPRAFILM

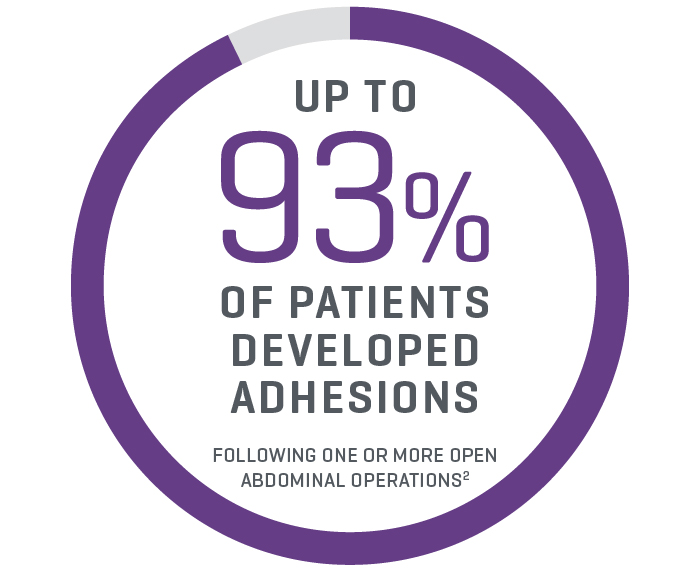
Adhesions are not preventable by surgical technique alone
Adhesions develop routinely following both open and laparoscopic* abdominal surgery, and have been reported at second-look surgery to occur in up to 93% of patients (n=210) following one or more open abdominal operations.3

The clinical and economic burden of adhesions is extensive
Adhesions are a major cause of small bowel obstruction, infertility, chronic pelvic pain, and can complicate future surgery, with annual surgical costs of more than $2 billion in the U.S.4,5
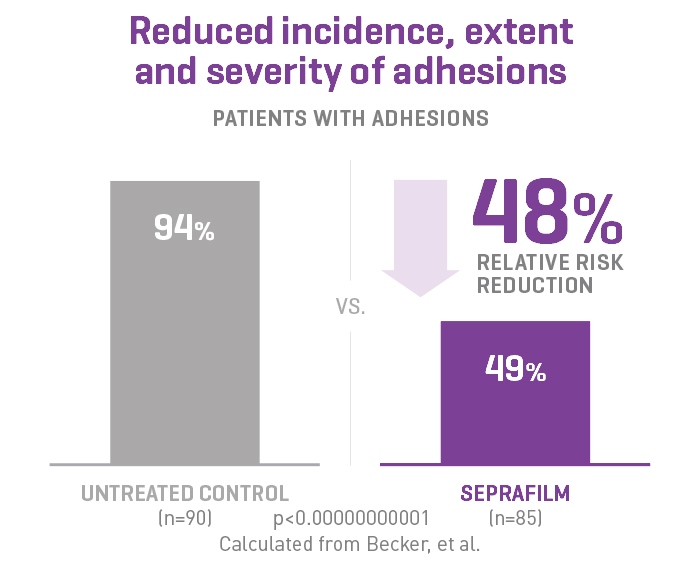
SEPRAFILM Adhesion Barrier efficacy in abdominal surgery
In a randomized, prospective, double-blinded, multicenter clinical study involving 183 patients [175 evaluable] with ulcerative colitis and familiar polyposis undergoing 2-stage intestinal resection, 51% of the Seprafilm Adhesion Barrier group were adhesion-free at 8-12 weeks compared to 6% of control patients.2
SEPRAFILM Adhesion Barrier is the only adhesion prevention product approved in the US for abdominal open procedures6
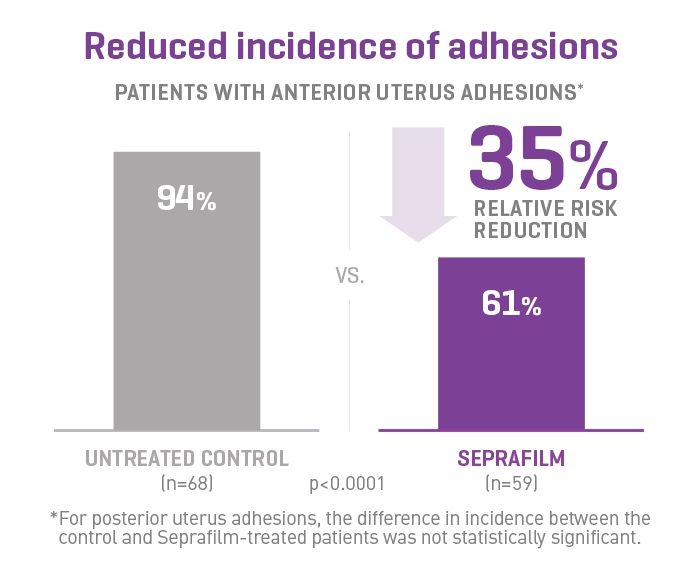
SEPRAFILM Adhesion Barrier efficacy in pelvic surgery
In a prospective, randomized, blinded, multicenter clinical study involving 127 patients undergoing gynecologic surgery, Seprafilm Adhesion Barrier reduced the mean number of sites adherent to the uterine surface following myomectomy compared with untreated patients. Seprafilm Adhesion Barrier also significantly reduced the extent and severity of adhesions in patients undergoing uterine myomectomy compared with untreated patients.7
Mechanism of Action
Seprafilm Adhesion Barrier is a sterile, bioresorbable, translucent and hydrophilic adhesion barrier. It is composed of two anionic polysaccharides: modified sodium hyaluronate and carboxymethylcellulose (HA/CMC).1
Additional Product Benefits
Protection When it Matters Most
Separates tissues for up to 7 days - the critical tissue healing period1
A Proven Legacy
More than 4 million patients have received Seprafilm Adhesion Barrier in clinical use worldwide.8 Robust clinical trials (n=2,133) across five clinical studies resulted in Seprafilm Adhesion Barrier significantly reducing the incidence, extent, and severity following abdominopelvic laparotomy surgery1,8
Bioresorbable and Synthetic
Seprafilm Adhesion Barrier is composed of sodium hyaluronate and carboxymethylcellulose (HA/CMC)1
SEPRAFILM Indications and Important Safety Information
INDICATIONS
Seprafilm Adhesion Barrier is indicated for use in patients undergoing abdominal or pelvic laparotomy as an adjunct intended to reduce the incidence, extent, and severity of postoperative adhesions between the abdominal wall and the under-lying viscera such as omentum, small bowel, bladder, and stomach, and between the uterus and surrounding structures such as tubes and ovaries, large bowel, and bladder.
IMPORTANT RISK INFORMATION
Seprafilm Adhesion Barrier is contraindicated in patients with a history of hypersensitivity to Seprafilm and/or to any component of Seprafilm.
Seprafilm Adhesion Barrier is contraindicated for use wrapped directly around a fresh anastomotic suture or staple line; as such use increases the risk of anastomotic leak and related events (fistula, abscess, leak, sepsis, peritonitis).
Seprafilm Adhesion Barrier must be used according to the instructions for use. Seprafilm Adhesion Barrier is supplied sterile and must not be re-sterilized. Seprafilm Adhesion Barrier is for single use only.
Every opened and unused Seprafilm pouch must be discarded. Do not use product if pouch is damaged or opened.
The number of sheets used should be just adequate to cover the under surface of the abdominal wall or uterine incision in a single layer.
In patients who have ovarian, primary peritoneal or fallopian tube malignancies, Seprafilm Adhesion Barrier use has been reported to have an increased risk of intra-abdominal fluid collection and/or abscess, particularly when extensive debulking surgery was required.
The safety and effectiveness of Seprafilm Adhesion Barrier has not been evaluated in clinical studies for the following: Patients with frank infections in the abdominopelvic cavity; patients with abdominopelvic malignancy, such as device use on resected liver surfaces following hepatectomy for malignant liver tumors; device placement in locations other than directly beneath an abdominal wall incision following laparotomy, or directly on the uterus following open myomectomy (not laparoscopic); patients with ongoing local and/or systemic inflammatory cell responses; device use in the presence of other implants, e.g. surgical mesh; patients requiring re-operation within four weeks of Seprafilm Adhesion Barrier placement – during anticipated time of peak adhesion formation. Foreign body reactions have occurred with Seprafilm Adhesion Barrier.
The safety and effectiveness of Seprafilm Adhesion Barrier in combination with other adhesion prevention products and/or in other surgical procedures not within the abdominopelvic cavity have not been established in clinical studies.
The safe and effective use of Seprafilm Adhesion Barrier in pregnancy and Cesarean section has not been evaluated. No clinical studies have been conducted in pregnant women or women who have become pregnant within the first month after exposure to Seprafilm Adhesion Barrier. Therefore, this product is not recommended for use during pregnancy and avoidance of conception should be considered during the first complete menstrual cycle after use of Seprafilm Adhesion Barrier.
Long term clinical outcomes such as chronic pain and infertility have not been determined in clinical studies.
Rx Only. For safe and proper use of this device refer to the complete Instructions for Use.