The Cost and Value of FLOSEAL Matrix in Gynecologic Surgery
O.R. Insights Blog
THE COST AND VALUE OF FLOSEAL MATRIX IN GYNECOLOGIC SURGERY
Perioperative blood loss is a recognized challenge in gynecological procedures, often complicating both the procedure and subsequent recovery by disrupting the operation, impairing organ exposure, prolonging the surgery and duration of hospitalization, increasing the need for transfusion and negatively impacting the cost of healthcare.1 In gynecological patients, blood transfusions are associated with increased surgical wound infections and composite morbidity and mortality.1
This article highlights the value of Floseal Matrix in gynecologic surgery by discussing the potential advantages over electrocautery when used near the ovary as well as in reducing bleeding-related complications and costs. Join us in exploring the intricate balance between cost, care and clinical outcomes in the context of gynecologic surgeries. Kate O’Hanlan, MD† of the Laparoscopic Institute for Gynecology and Oncology (LIGO) in Portola Valley, California, will provide an overview of the economic burden of bleeding in gynecologic surgery and the potential cost savings as a result of the utilization of active hemostatic agents such as Floseal Matrix.
Introduction
Inadequate surgical hemostasis may lead to transfusion and/or other bleeding-related complications.2 Therefore, early control of surgical bleeding is paramount.3 While securing hemostasis with cautery or suture, care must be taken to avoid tissue necrosis, organ injury, vascular thrombosis, fistula formation and/or nerve dysfunction.4 In laparoscopic surgery, hemostasis can be particularly challenging because intracorporeal suturing may be too slow or cumbersome.3 Topical hemostatic agents are thus useful adjuncts to conventional hemostatic modalities.3
Approximately 4 million
gynecologic surgeries are performed in the United States annually, with nearly 1.5 million performed in the inpatient setting5
Bleeding-related complications
occur in approximately 7.5% of gynecologic procedures with a significant impact on hospital costs2
Key Differences Between Active and Passive Hemostatic Agents
Topical hemostatic agents have been classified in different ways based on their mechanism of action. One of the most widely used classifications for hemostatic products is based on whether the product provides a physical structure around which platelets can aggregate so a clot can form (passive) or whether the product delivers their mechanism of action on the clotting cascade in a biologically active manner (active) (Figure 1).6
Passive products (e.g. oxidized cellulose, collagen, gelatin, etc.), or mechanical hemostats, provide a physical structure for platelet activation and clot formation.6 Further, they must rely on the patient’s own quantity and quality of platelets and the patient’s clotting factors.6 Passive products are most effective for minimal bleeding scenarios and are appropriate for patients who have an adequately functioning coagulation cascade.6

Figure 1. Active and Passive Topical Hemostatic Agents6
Active products (e.g., topical thrombin, fibrin sealants, etc.) contain thrombin, an active ingredient that participates at the end of the coagulation cascade.6,7 Thus, active products are effective in patients who may have coagulopathies inhibiting other portions of their own coagulation cascade.6 Patients with platelet dysfunction, coagulopathies due to abnormal or low clotting factors (other than hypofibrinogenemia), or those receiving antithrombotic medications may achieve successful hemostasis with active hemostats.6
What is Floseal Matrix?
Floseal Matrix is composed of proprietary cross-linked bovine-derived gelatin granules combined with thrombin that conform to and swell at the bleeding site to provide a tamponade effect to help control bleeding.7,8 In vitro studies have shown that the surfaces of the discrete Floseal Matrix granules are smooth which increases its ability to retain thrombin and red blood cells.9
When the Floseal Matrix granules are hydrated with recombinant thrombin and applied to a bleeding site, the patient’s fibrinogen is converted into fibrin monomers and polymers, initiating and accelerating clot formation (Figure 1).8 Because Floseal Matrix contains thrombin, it impacts the clotting cascade in a biologically active fashion, and is considered an active hemostatic agent. Active agents function at the end of the coagulation mechanism bypassing the need for intact platelet function and can be successfully employed in patients receiving antithrombotic medications.6
Selected Risk Information for Floseal Matrix: Do not administer to patients with a history of hypersensitivity to Recothrom thrombin, any components of Recothrom, or hamster proteins. Do not use Floseal Matrix in patients with known allergies to materials of bovine origin.
Figure 2. Floseal Matrix Mechanism of Action7,8
Selected Risk Information for Floseal Matrix: Do not inject Floseal Matrix into blood vessels. Do not apply Floseal Matrix in the absence of active bleeding.
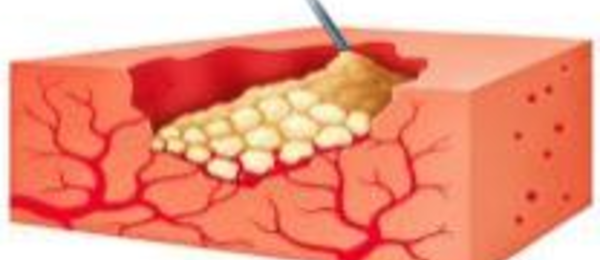
1. Floseal Matrix is applied to the tissue surface at the base of the lesion; its granules fill the wound and conform to its shape.
2. Floseal Matrix granules expand approximately 10-20% within about 10 minutes and physically restrict the flow of blood. Blood percolates through the spaces and is exposed to thrombin.

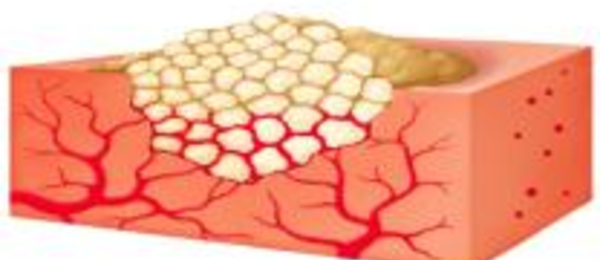
3. A clot forms around the mechanically stable matrix provided by the granules. The structural integrity of the gelatin fibrin matrix enables it to remain in place at the tissue surface.
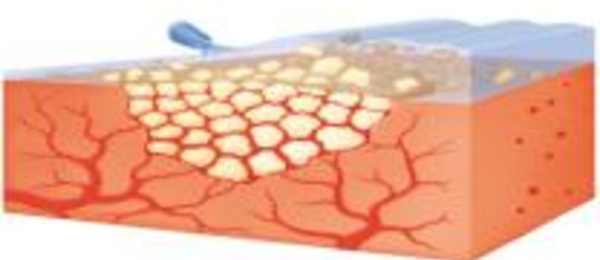
4. Once hemostasis is achieved, excess Floseal Matrix should always be removed by gentle irrigation and suctioned out of the wound.
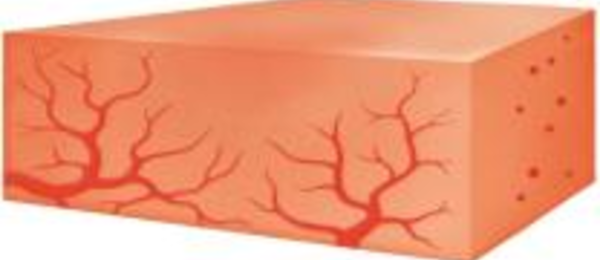
5. Floseal Matrix is resorbed by the body within 6-8 weeks, consistent with the time frame of normal wound healing.
With the unique combination of patented gelatin granules and recombinant thrombin, Floseal Matrix provides fast (2-minute median time), effective hemostasis.7,8 To learn more about how Floseal Matrix helps bleeding control in critical gynecologic surgeries, the case study below demonstrates the efficacy and speed of Floseal Matrix in achieving rapid hemostasis in the management of an avulsed artery (Video 1).
Video 1. Floseal Matrix: Gynecologic Surgery Case Study
Use of a Validated Bleeding Scale
The use of a validated bleeding scale could ultimately improve surgical efficiency by selecting the right hemostatic agent for the right bleed.10 The VIBe Scale tool (Validated Intraoperative Bleeding Scale) is the first surgeon-validated scale accepted by the United States (US) Food and Drug Administration (FDA) designed for consistent and reliable assessment of intraoperative bleeding severity (Figure 3). 10

Figure 3. VIBe Scale Tool for Consistent, Reliable Assessment of Intraoperative Bleeding10,11
Overview of FLOSEAL Matrix in Gynecologic Surgery in Preserving Ovarian Function
Several studies have reviewed the use of Floseal Matrix in ovarian cystectomy to assess reduction of blood loss.3,12,13 Angioli et al3 were the first to investigate the effectiveness of Floseal Matrix during laparoscopic endometrioma excision. Hemostasis was achieved in all patients within 3 minutes with a median time of 172 and 182 seconds.3
Floseal Matrix was also compared with electrocautery in three randomized controlled trials (RCTs) also addressing endometriomas. Sönmezer et al12 evaluated anti-müllerian hormone (AMH) levels, used to assess a woman’s ovarian reserve. Compared with preoperative AMH levels, the AMH levels one month after surgery were significantly more decreased in the bipolar electrocautery group than the Floseal Matrix group (56% vs. 29%, respectively, p=0.001).12 However, the AMH levels were increased and became similar at the third postoperative month.12 Further studies, with long-term follow-up, will clarity the importance of these findings.12
In another RCT with 100 patients, Song et al13 compared AMH levels between Floseal Matrix and bipolar electrocautery at the third postoperative month following laparoscopic excision of endometriomas. Again, the percentage AMH levels had a significantly greater decrease in the bipolar electrocautery group vs. Floseal Matrix (41.2% vs. 16.1%, respectively; p=0.004).13 Both the Sonmezer et al12 and Song13 state that larger scale, long-term follow-up, randomized controlled trials are needed to conform, or reject, the results of these studies.
In the third RCT, by Chung et al14, the primary outcome was the effect on the antral follicular count (AFC) at the third postoperative month. The AFC refers to the number of follicles with diameters of 2 mm to 9 mm; these follicles generally reflect the number of follicles that will continue to mature during the ovulation cycle.15 The increase in AFC of the affected ovaries at three months in the Floseal Matrix group was significantly higher than that in the bipolar electrocautery group (2.36±0.37 vs. 1.08±0.36, respectively; p=0.013).14
Clinical Outcomes and Economic Benefits of Floseal Matrix
Despite the growing evidence of the clinical and economic outcome benefits of active products, many surgeons typically employ a passive product as the first choice surgical adjunct to regaining hemostasis.6 A pattern of repeated, unsuccessful use of a passive agent is often seen before an active agent is employed to successfully achieve hemostasis.6
While the cost of Floseal Matrix may vary based on location, volume, and procurement contracts, it is imperative to consider this modest investment against the backdrop of significantly improved hemostasis and economic outcomes as compared with passive agents.6
The economic impact reflects both direct and indirect healthcare costs.6 A study by Iannitti and colleagues6 utilized a large database to compare the outcomes and associated costs of surgeries employing active agents solely versus a combination of passive and active that demonstrated clear financial benefits favoring the use of active agents initially and alone.6 Many surgeons initially employ a passive product, sometimes repeatedly, regardless of the ineffectiveness of the hemostat.6
Results by Iannitti demonstrated that surgeries using active agents such as Floseal Matrix, could be successfully employed alone, with notably lower total hospital costs and shorter lengths of stay.6 Specifically, in the realm of reproductive surgeries, the adjusted total hospital costs and bleeding-related complications were significantly lower for the cohort using active agents only (Table 1).6

Table 1: Economic Impact of Hemostatic Agents in Reproductive Surgery6
Note: Costs and percentages are derived from adjusted data, reflecting differences attributable to the use of active vs. combined hemostatic agents. Percentages are calculated to show the relative decrease when using active agents only.
The economic evaluation included both unadjusted and adjusted analyses of total hospital costs.6 The unadjusted mean total hospital costs for patients treated with active agents were consistently lower compared to those receiving both passive and active agents.6 After adjusting for potential confounders such as demographics, baseline clinical characteristics and hospital characteristics, the difference remained statistically significant.6 This suggests that the use of active hemostatic products like Floseal Matrix may lead to a substantial reduction in the financial burden on healthcare facilities.6
Moreover, the study highlights that the adjusted odds of experiencing bleeding-related complications were also significantly lower in the active-only agent group.6 This reduction in complications supports better patient outcomes and also reduces the need for additional interventions or prolonged hospitalization, further driving down costs.6
Summary
In gynecologic surgeries, controlling perioperative bleeding is crucial not only to improve the visibility of the surgical field but also to mitigate risks such as extended hospital stays and increased transfusion needs.3,6 Several studies have also demonstrated that use of Floseal Matrix in gynecologic surgery as compared with electrocautery may contribute to the preservation of ovarian reserve.3,12,13 Floseal Matrix, an active hemostatic agent, plays a pivotal role by effectively managing bleeding, thereby reducing the perioperative blood loss that complicates surgeries and inflates healthcare costs.6,8 Studies, including those by Iannitti and colleagues,6 demonstrate that the use of active agents, such as Floseal Matrix alone, results in successful hemostasis, and is associated with significant cost savings by reducing total hospital costs as well as the incidence of bleeding-related complications compared to passive agents. These savings are underscored by the substantial decrease in both direct and indirect healthcare expenses, highlighting the capacity of Floseal Matrix to enhance clinical outcomes and optimize economic benefits in gynecologic surgery.6
†Dr. Kate O’Hanlan was compensated for her time developing the content for this blog.
FLOSEAL HEMOSTATIC MATRIX INDICATIONS AND IMPORTANT RISK INFORMATION
INDICATIONS
Floseal Matrix is indicated in surgical procedures (other than ophthalmic) as an adjunct to hemostasis when control of bleeding by ligature or conventional procedures is ineffective or impractical.
Important Risk Information
Do not inject intravascularly.
Do not inject or compress Floseal Matrix into blood vessels.
Do not apply Floseal Matrix in the absence of active blood flow, e.g., while the vessel is clamped or bypassed, as extensive intravascular clotting and even death may result.
Do not administer to patients with a history of hypersensitivity to Recothrom Thrombin Topical (Recombinant), any components of Recothrom, or hamster proteins.
Do not use Floseal Matrix in patients with known allergies to materials of bovine origin.
Do not use Floseal Matrix in the closure of skin incisions because it may interfere with the healing of the skin edges due to mechanical interposition of gelatin.
Floseal Matrix is not intended as a substitute for meticulous surgical technique and the proper application of ligatures or other conventional procedures for hemostasis. Floseal Matrix is not intended to be used as a prophylactic hemostatic agent.
As with other hemostatic agents, do not apply Floseal Matrix to sites where there is negative peripheral venous pressure, as material may be drawn into the vascular system potentially resulting in life threatening thromboembolic events.
Excess Floseal Matrix (material not incorporated in the hemostatic clot) should always be removed by gentle irrigation and suctioned out of the wound.
The particles of Floseal Matrix swell approximately 10-20% (upon contact with blood or other fluids) and surgeons should consider its potential effect on the surrounding anatomic areas. Maximum swell volume is achieved within about 10 minutes.
Floseal Matrix should not be used in the presence of infection. Floseal Matrix should be used with caution in contaminated areas of the body.
The safety and effectiveness of Floseal Matrix for use in ophthalmic procedures has not been established.
Floseal Matrix should not be used for controlling post-partum bleeding or menorrhagia.
The safety and effectiveness of Floseal Matrix has not been established in children under 2 years of age and pregnant women.
It is not known whether Floseal Matrix can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Floseal Matrix should be administered to a pregnant woman only if clearly needed.
Do not use air to remove residual Floseal Matrix from Applicator tip. The Applicator tips should not be cut, as tissue injury from sharp edges may result.
Hypersensitivity reactions, including anaphylaxis, may occur. Recothrom thrombin is produced in a genetically modified Chinese Hamster Ovary (CHO) cell line and may contain hamster or snake proteins.
For single use only. Do not re-sterilize.
Floseal Matrix should not be applied before the application site is cleaned to remove any antiseptics that may contain alcohol, iodine, or heavy metal ions.
When placed into cavities or closed tissue spaces, gentle approximation is advised. Do not compress.
As with other hemostatic agents, do not aspirate Floseal Matrix into extracorporeal cardiopulmonary bypass circuits or autologous blood salvage circuits.
Do not use Floseal Matrix on bone surfaces where adhesives, such as methylmethacrylate or other acrylic adhesives, will be required to attach a prosthetic device.
Floseal Matrix should not be used for the primary treatment of coagulation disorders.
The safety and effectiveness of the combined use of Floseal Matrix with antibiotic solutions or powders has not been established.
The safety and effectiveness for use in neurosurgical and urological procedures has not been established through randomized clinical studies.
In urological procedures, Floseal Matrix should not be left in the renal pelvis or ureters to eliminate the potential foci for calculus formation.
Antibody formation to Recothrom thrombin occurred in <1% of patients. None of the antibodies detected neutralized native human thrombin.
Thrombin must be added to the Gelatin Matrix prior to use.
Rx Only. For safe and proper use of this device, refer to the full Instructions for Use.
Baxter, Floseal, Recothrom and VIBe Scale are registered trademarks of Baxter International Inc.