What is Adept Adhesion Reduction Solution?
O.R. Insights Blog
What is Adept Adhesion Reduction Solution?
Post-surgical adhesions, bands of connective tissue that join two normally separate anatomical structures, are one of the most common sequelae of gynecological surgeries, leading to chronic pain, infertility or bowel obstruction.1 The extent of the problem of adhesions has been underestimated by surgeons and the health authorities, but there is rising evidence that surgeons can take important steps to reduce the impact of adhesions.1 Among them, Adept Adhesion Reduction Solution is a broad coverage adhesion reduction agent that is designed for application during laparoscopic procedures as an adjunct to good surgical technique.2
This article will provide an overview of Adept Adhesion Reduction Solution, a 4% icodextrin solution, by exploring its mechanism of action, key clinical findings, and providing guidance on use. Whether you're already familiar with this adhesion barrier or hearing about it for the first time, Mona Orady, MD, Past President of the Society of Laparoscopic and Robotic Surgery and Director of Robotic Surgery at St. Francis Memorial Hospital*, will outline the crucial role it plays in minimally invasive gynecologic surgery.
Introduction
The formation and reformation of post-surgical adhesions lead to significant patient morbidity such as small bowel obstruction, infertility, chronic pain, and difficult, complicated subsequent surgeries.3-5 Pre-existing adhesions significantly prolong the duration of surgery and lead to considerable complications in an important percentage of patients.6 Pelvic adhesions are the most common cause of infertility and chronic pelvic pain.7
Various strategies have been put forth to prevent adhesion development including meticulous surgical techniques, pharmacological agents and new equipment and instrumentation.4 Adept Adhesion Reduction Solution is a 4% icodextrin solution that acts as a hydroflotation fluid reservoir that effectively separates damaged peritoneal surfaces providing a barrier to adhesion formation.2,8
ADEPT Adhesion Reduction Solution is the only fluid-based solution for gynecological adhesion reduction approved by the United States Food and Drug Administration. 2
Adept Adhesion Reduction Solution provides broad coverage and optimized resorption: icodextrin slows the resorption time while other fluids are resorbed much faster.2,4,8
What is ADEPT Adhesion Reduction Solution?
Adept Adhesion Reduction Solution received approval from the United States (US) Food and Drug Administration (FDA) in 2006 and is available in North America and worldwide.8,9 It is a single use, sterile, clear, colorless-to-pale yellow fluid for intraperitoneal administration containing icodextrin at a concentration of 4% w/v in an electrolyte solution.8 Icodextrin is a cornstarch-derived, water-soluble branched glucose polymer.8 Adept solution is packaged in flexible polyvinylchloride bags containing 1 L or 1.5 L of solution.8
Selected Risk Information for Adept solution: Do not use in patients with known or suspected allergy to cornstarch-based polymers e.g. icodextrin, or with maltose or isomaltose intolerance or with glycogen storage disease.8
What is the Indication?
Adept Adhesion Reduction Solution is indicated for use intraperitoneally as an adjunct to good surgical technique for the reduction of post-surgical adhesions in patients undergoing gynecological laparoscopic adhesiolysis.8
How Does It Work?
The use of fluids in the peritoneal cavity to separate damaged peritoneal surfaces and prevent contact between organs during the time of postoperative repair has been proposed as a method of adhesion reduction—a process known as hydroflotation.2 Icodextrin, as an alpha (14) linked glucose polymer, is similar in structure to carbohydrates which occur physiologically, e.g. glycogen.8 When administered intraperitoneally as a 4% solution, icodextrin functions as a colloid osmotic agent. This colloidal osmotic action of icodextrin allows the retention of a reservoir of fluid within the peritoneal cavity for 3-4 days.8
During laparoscopic gynecological surgery, Adept solution may be used as an irrigant solution during the course of surgery.8 Once the surgical procedure is completed, and the cavity is aspirated of all remaining fluid, a final volume of 1 liter of Adept solution is then introduced into the cavity before removal of the scope.8
Adept solution is believed to perform its function through a physical effect by providing a temporary separation of peritoneal surfaces by hydroflotation as a result of maintaining a fluid reservoir.8 This minimizes tissue apposition during the critical period of fibrin formation and mesothelial regeneration following surgery, thereby providing a barrier to adhesion formation.8
The peritoneal surface takes several days to recover after surgery, so solutions with a short residence time would not be expected to prevent adhesion formation.2 This is supported by the results of a meta-analysis of 22 published reports, which showed that the use of crystalloid instillates in volumes of up to 500 mL did not increase adhesion-free outcome in patients undergoing abdominopelvic surgery.10
Marked differences in intraperitoneal residence times between icodextrin (which is absorbed slowly over a period of several days) and crystalloid solutions (which remain in the cavity for approximately 24 hours) support the idea that prolonged hydroflotation helps to keep peritoneal surfaces apart during the key stages of wound repair, thus reducing the likelihood of adhesion formation.3
Clinical Evidence for ADEPT Adhesion Reduction Solution
Clinical Evidence: Pivotal Study
In the pivotal study for Adept Adhesion Reduction Solution known as PAMELA (Pivotal, Double-Blind, Comparative, Multicenter Study to Determine the Efficacy and Safety of ADEPT Adhesion Reduction Solution in Reduction of Post Surgical Adhesions after Laparoscopic Surgery), the object was to evaluate the safety and efficacy in reducing adhesions after laparoscopic gynecologic surgery involving adhesiolysis as compared to lactated Ringer’s solution (LRS).2 The methodology involved initial adhesion scoring and follow-up laparoscopy 4–8 weeks later, assessing the presence, extent, and severity of adhesions, with clinical success defined as a reduction in adhesions of at least 3 or 30% of sites lysed.2
Results demonstrated a significant reduction in adhesion formation with Adept Adhesion Reduction Solution as compared to LRS, with 49% of the Adept solution patient achieving clinical success versus 38% in the LRS group (Figure 2).2 Safety profiles were similar between both groups, indicating Adept solution is an effective and safe option for reducing adhesions following gynecologic laparoscopic adhesiolysis.2
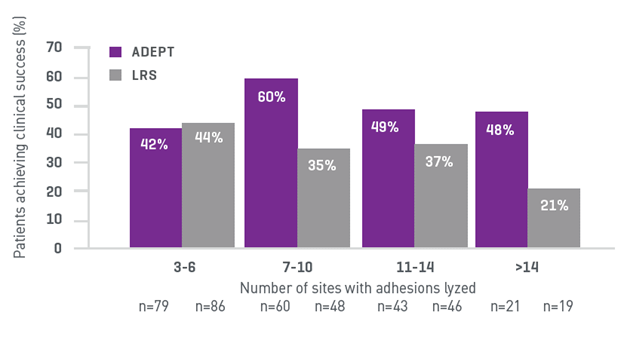
Figure 2. Clinical Success of Adept Adhesion Reduction Solution versus Lactated Ringers in the PAMELA Study2
Clinical Evidence: ARIEL Patient Registry
The ARIEL (ADEPT Registry for Clinical Evaluation) Registry was established in 2000 to provide systematic feedback on the use of Adept Adhesion Reduction Solution in routine gynecological surgery.11 Gynecological surgeons from 150 centers recorded patient demographics, use of Adept Adhesion Reduction Solution and adverse events, and made subjective assessments of ease of use and patient acceptability with the agent.11 The gynecological surgery registry included 2,882 patients; 72% (n=2,069) underwent laparoscopies.
Most surgeons rated the ease of use (viewing of surgical field and handling of tissues) as ‘excellent’ or ‘good’.11 The incidence of adverse events reflected expected rates in gynecological surgery.11 ARIEL data indicate that Adept Adhesion Reduction Solution was well tolerated and easy to use for the reduction of adhesion formation following gynecological surgery.11
I've done over 1000 cases of endometriosis excisions, which is notorious for causing pelvic adhesions as well as myomectomy and ovarian cystectomy in patients who wish to maintain fertility. Because fertility preservation is so important in these patients, I am meticulous about reducing adhesions. In addition to good surgical technique and meticulous hemostasis, I have started consistently using Adept Adhesion Reduction Solution. In my experience, on reoperation I’ve found reduction of post-surgical adhesions in patients undergoing laparoscopic adhesiolysis.8 That has been my experience reoperating on my patients as compared to reoperating on patients where adhesion reduction wasn't a priority.
Mona Orady, MD, St. Francis Memorial Hospital
Click Play to learn more about Dr. Orady’s surgical and adhesion prevention techniques.
Use of ADEPT Adhesion Reduction Solution: Patient Information
Baxter has developed a useful patient brochure titled, ”The Simple Solution for Reducing Adhesions.” Preparing your patients to better understand how Adept Adhesion Reduction Solution works and its post-surgical effects, may help alleviate their concerns. While some side effects, such as vulvar swelling, may occur,8 these are minor compared to the health risks associated with adhesions, such as chronic pain, infertility, small bowel obstruction and potential complications in subsequent surgeries.3-5 By remaining in the abdomen for 3-4 days post-surgery, Adept solution helps minimize adhesion formation during this critical healing period.8 It's essential to communicate to patients that, although adhesions may not be entirely preventable, adopting adhesion reduction strategies is important.2,8 This patient education will empower your patients to make informed decisions about their care.
Summary
In summary, Adept Adhesion Reduction Solution represents an important advancement in the reduction of post-surgical adhesions, a common complication in abdominopelvic surgeries.1 Composed of icodextrin 4% solution, Adept Adhesion Reduction Solution Barrier prevents contact between organs during the time of postoperative repair in a process known as hydroflotation.2
Clinical evidence has demonstrated the effectiveness of Adept Adhesion Reduction Solution in reducing post-surgical adhesions following minimally invasive gynecological surgery that involves adhesion release.2 A key aspect of Adept solution is its ease of use which most surgeons rated as ‘excellent’ or ‘good’.11 In addition, it is the only fluid-based solution for gynecological adhesion reduction approved by the US FDA.2 By offering a reliable option to a longstanding challenge in surgery, Adept Adhesion Reduction Solution supports patient recovery and exemplifies the progress in surgical adhesion reduction.8
*Dr. Mona Orady was compensated for her time developing this blog.
ADEPT® Adhesion Reduction Solution [4% Icodextrin] Indications
ADEPT® Adhesion Reduction Solution is indicated for use intraperitoneally as an adjunct to good surgical technique for the reduction of post-surgical adhesions in patients undergoing gynecological laparoscopic adhesiolysis.
Important Risk Information for ADEPT®
ADEPT® Solution is for direct intraperitoneal administration only. NOT for intravenous (IV) administration.
ADEPT® is contraindicated in patients with known or suspected allergy to cornstarch based polymers e.g. icodextrin, or with maltose or isomaltose intolerance, or with glycogen storage disease.
ADEPT® is contraindicated in laparotomy, in cases involving bowel resection or repair, or appendectomy and in surgical cases with frank abdomino-pelvic infection.
There have been rare reports of sterile peritonitis following the use of icodextrin.
Leakage of ADEPT® from port sites may lead to wound healing complications; meticulous fascial closure may reduce leakage through laparoscopic port sites post-operatively.
There have been rare reports of hypersensitivity reactions, pulmonary edema, pulmonary effusion and arrhythmia.
Anaphylaxis has been reported in a few patients.
Maltose metabolites of icodextrin may interfere with blood glucose measurement in diabetic patients who use rapid blood glucose systems that are not glucose specific.
In the pivotal study, the most frequently occurring treatment related adverse events between surgeries were post procedural leaking from port sites, labial, vulvar or vaginal swelling and abdominal distention.
Rx Only. For safe and proper use of this device, please refer to full Instructions For Use.